Special guest blog post by Marissa Carter, PhD
Predicting Healing on the First Visit (for venous ulcers and other wound types)
There is a lot of interest in “predictive models” for chronic wounds and ulcers. The ideal predictive model can predict at the FIRST VISIT whether a wound will heal (assuming standard care). It is a big disadvantage to have to wait 4 weeks for more wound measurements to make this prediction. A major reason we need a model that works on “day one” is that payers would like to use predictive models to decide whether certain chronic wounds even need to be referred to a wound center (for example, if they are highly likely to heal, they would like the primary care physician to treat them). Conversely, wounds predicted NOT to heal could be identified immediately for advanced therapeutics, without having to undergo a month of futile care. Although the ideal predictive model can run at the initial visit, these “first visit” models can be limited if certain critical data points are not known at the initial visit. This is one reason that the arterial screening quality measure for patients with leg ulcers requires that arterial screening be performed AT THE FIRST VISIT.
Another challenge with regard to predictive models (developing and validating them) is that real-world patients typically require long periods of time to heal. Data from the US Wound Registry (USWR) confirms that the average patient stays in service for nearly 8 months. In fact, the clinicians reading this know that many patients attend wound centers for longer than a year. Over such long treatment periods, patients can be lost to follow up, transferred to another provider, die, move, become residents of long-term care facilities, and even decide to be seen at another wound center. That makes it challenging to develop models if you are missing the outcome of a lot of wounds. Even with these major limitations, we were able to create reliable models that can predict whether a wound will heal based on the information available at the first visit. The Wound Healing Index (WHI) allows us to predict with 75-80% accuracy whether a wound will heal at the conclusion of the first clinic visit. This makes the WHI superior to models that require observation over time.
Simple vs. Complex Patients and Wounds
Clinicians know that patients and their wounds are a very heterogeneous group, even if we look at one particular wound “type”. One of the ways we can start to classify them is by thinking of wounds in terms of whether they are simple or complex wounds and thinking of hosts (patients) as simple vs. complex. For example, a “simple venous leg ulcer (VLU)” might be small (e.g., <10 square cm) and “young” (e.g. only 2 months old when evaluated) whereas a “complex VLU” might be much larger (perhaps 75 square cm), and very old (>1 year). A “simple patient” might have no major co-morbid conditions, whereas a “complex patient” may have congestive heart failure, sleep apnea, and be taking long-term cancer immunotherapy. We can visualize the combinations in Figure 1 below. Note most of the patients and their wounds who are enrolled into randomized controlled trials (RCTs) are in the simple wound/simple host box.
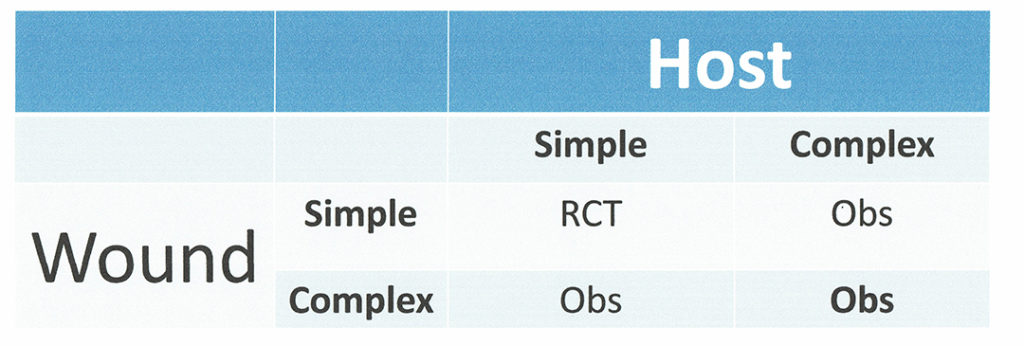
Creating a Risk Stratification for Wounds
We now need to layer our 2×2 simple/complex wound/host table over a continuous scale using a risk stratification algorithm. “Risk stratification” is a means of predicting or identifying patients most likely to experience a specific outcome. In this case, we are assessing the likelihood of healing or non-healing. The algorithm we’re going to use calculates the probability of wound healing. It is called the Wound Healing Index (WHI) and was originally developed 7 years ago in 2013. WHI calculations exist for several different wound types (diabetic foot ulcers, venous leg ulcers, pressure ulcers on the heels, pressure ulcers on the torso, dehisced surgical wounds and traumatic wounds). We will focus on VLUs (1).
The WHI is a continuous variable, but it is clinically easier to use if we create 3 categories or “buckets”: (1) Patients and their VLUs that will generally heal with good standard of care; (2) Patients and their VLUs that may or may not heal depending on the care provided (and that would likely benefit and/or require advanced therapeutics); and (3) Patients and their VLUs which are unlikely to heal. These patient/VLU combinations may either require very long time frames to heal, or may be poor candidates for advanced therapeutics because we have virtually no information on their effectiveness in such compromised patients/wounds. Category 1 represents simple host/simple wounds, category 2 the combinations of simple host/complex wound and complex hist/simple wound, and category 3 the complex host/complex wound.
How do we create the necessary breakpoints on the WHI scale, which runs from 0 (no chance of healing) to 100 (will definitely heal)? We need to bear 2 things in mind: the first is that each category has to be a reasonable percentage of the total, which means from statistician’s point of view, at least 5%. Second, we want a means of determining the probability of healing of categories (2) and (3) if we use category (1) as a reference group. This part can be solved by using a logistic regression, the details of which we won’t go into since this is a blog for clinicians and not statisticians!
Creating “Healing Likelihood” Categories for Wounds – the Venous Leg Ulcer example
Using the VLU WHI as an example, and doing a lot of iterative “tweaking” (based on real patients and real healing data), we created the following WHI VLU categories:
(1) Patients/VLUs likely to heal: ≥76
(2) Patients/VLUs that could “go either way”: 55-75.99
(3) Patients/VLUs that are not likely to heal: <55
Based on analysis of approximately 65,000 VLUs, the rough percentages for these categories were 52.9% (likely to heal), 40.1% (could go either way), and 7% (aren’t likely to heal). The associated odds ratios (ORs) for categories (2) and (3) are 0.75 and 0.41, respectively. Odds (and odds ratios) and not the same as probability but they do tell us that (a) the odds of healing is less than 1 (less than the odds of healing in our reference category 1) and that (b) the odds of healing in category (3) are far worse than for category (2), as we might expect.
How to use the VLU WHI Predictive Categories
Now that we have created VLU WHI–based categories, we can see that 53% of VLUs are in Category (1), meaning, 53% of VLUs are likely to health without advanced therapeutics. It should be emphasized that if the wound is still not on a wound-healing trajectory after 2-3 months, obviously it would be time to re-think that option. It is also worth saying that there is little reason to “brag” on healing rates among the patients/VLUs that are expected to heal! It turns out that when you evaluate the subjects and VLUs enrolled in randomized, prospective trials (RCTs), about 80-85% of RCT subjects fall into the category of VLUs expected to heal, especially if the trials were conducted in the USA. Payers create coverage policy based on these trials, which means that coverage policies for advanced therapeutics are focused on the VLUs that can be expected to heal without them and thus exclude the 40% of patients who really need advanced therapeutics (and could also be helped by them).
Category (3) defines the patient/VLUs that are very unlikely to heal. This means that if you do manage to heal them, your performance as a clinician or the intervention(s) you used changed the outcome of these VLUs – and that is a big deal. Clinicians should not be “punished” for being unable to heal VLUs that probably can’t be healed in the first place.
It may be fair to say that Wounds in category (2) are the most interesting and perhaps most important category. These represent the VLUs that COULD heal if they get the right treatment, but won’t heal if they don’t. We should use the healing rate of VLUs in Category 2 to evaluate VLU quality of care. It is in Category 2 where we can see the biggest impact of clinical expertise and the biggest value for advanced therapeutics. Check out Figure 2.
Summary:
The VLU WHI was developed in 2013 (although the paper describing it was published in 2020). It can predict at the conclusion of the FIRST VISIT with good accuracy whether a patient with a VLU is likely to heal. The VLU WHI is the method approved by CMS for reporting the quality measure of VLU healing rate under the Merit Based Incentive Payment System (MIPS). It has been used for creating matched cohorts in both prospective and retrospective trials. VLU healing rates are best reported using the “3 Category” approach in which Category 2 allows the best assessment of clinical quality of care and the value of advanced therapeutics. The VLU WHI can be calculated if you know the answers to the questions in Table 1 below at the conclusion of the initial visit. A different calculation must be run for EACH of the patient’s VLUs, and the average patient has at least 3 VLUs!
Fig 2:
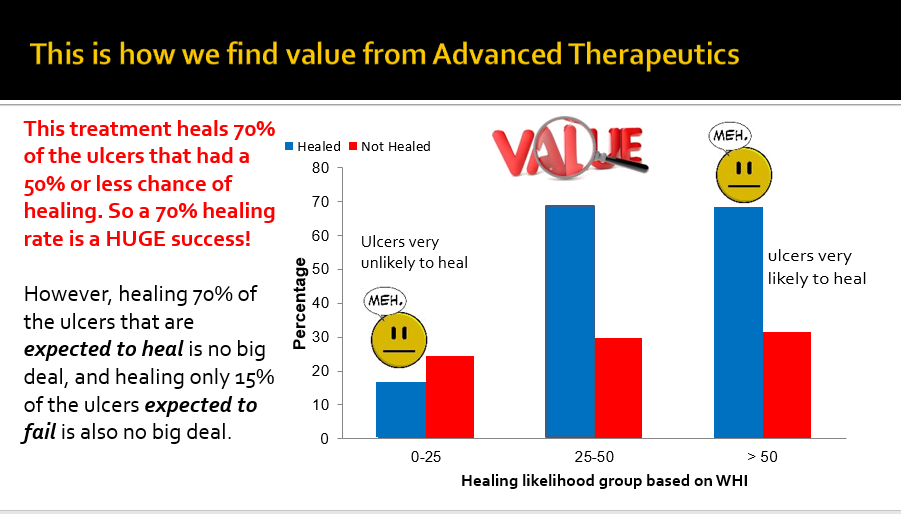
Table I. Significant variables used in predicting venous leg ulcer healing.a
| Variable |
|
|
|
|
|
|
|
|
|
| References
1. Fife CE, Horn SD. The Wound Healing Index for predicting venous leg ulcer outcome. Adv Wound Care (New Rochelle) 2020;9(2):68-77.
|

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.



As George Box said: All models are wrong, but some are useful. This model is useful!