There’s a “problem” with the proposed LCD on CTPs/skin subs that no one is talking about. It’s a problem we’ve largely ignored for more than 20 years. To explain, I have to go back to 1998 when I was one of several sites for both the Apligraf and the Regranex clinical trials. I’d been running a wound center for 8 years by then in an academic setting. I was young and very excited to be participating in what I thought were the first steps at harnessing the body’s own healing process. I did notice that I could not enroll any actual patients in the clinical trials I was doing. I had to go on radio talk shows and put ads in the newspapers — which is how clinical trials were advertised before the Internet . . .
Why does it matter that clinical trials enroll subjects that bear no resemblance to patients? It matters because it affects coverage policy for the products once they are on the market.
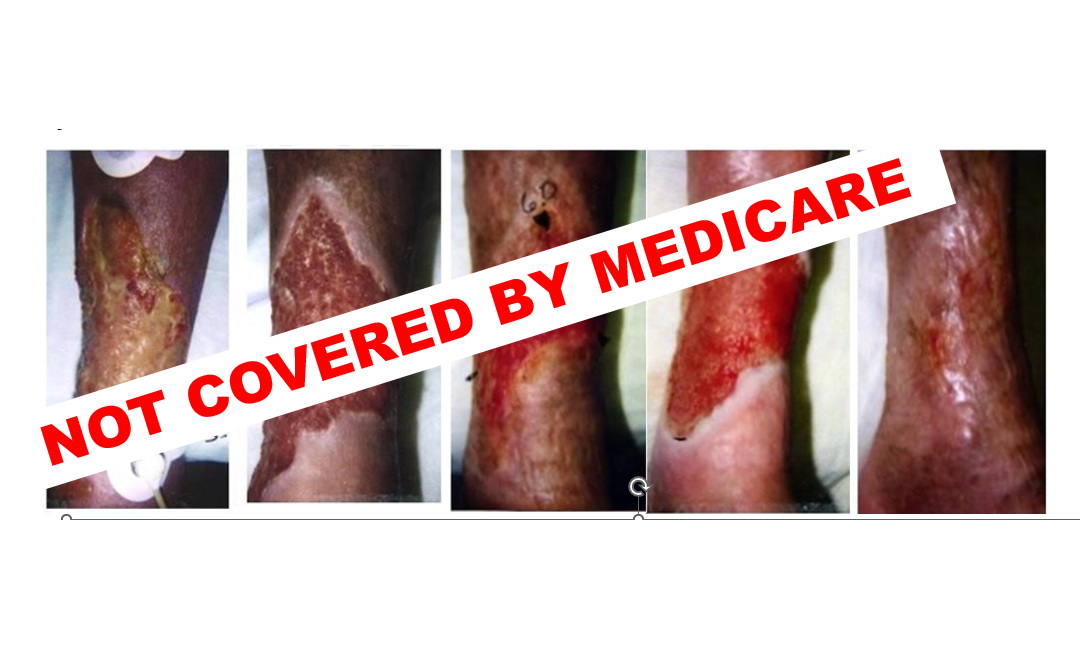
Here’s what happened. As soon as Apligraf was available on the market, I started using it in difficult non-healing wounds. I had also been using a brand-new technology called “The VAC”, so I was excited about being able to bring these huge advances to patients. However, the trajectory of my research career changed with this one patient. She was a 72-year-old woman with uncontrolled diabetes, peripheral arterial disease, and rheumatoid arthritis, on prednisone and Methotrexate. She probably had an inflammatory ulcer although I didn’t know how to diagnose it then. In the first photo you can see transcutaneous oxygen electrodes. I was evaluating all patients to determine whether they had enough perfusion to heal and was working with invasive cardiology to perform endovascular revascularizations rather than surgery, an approach that was still heresy at the time and for which I was soundly criticized by the vascular surgeons. This terrible wound healed with negative pressure wound therapy and Apligraf. I was so excited!
I thought I was providing the best care possible in the country, until I read the brand new policy for the use of “skin substitutes” issued by my Medicare Administrative Contractor (MAC), which at the time was Trailblazer. The Local Coverage Determination (LCD) said that I could only use a skin substitute for wounds “ . .. not involving tendon, muscle, joint capsule or exhibiting exposed bone or sinus tracts. . . .[only after] adequate debridement of necrotic tissue. . . .Only applied to wounds with adequate circulation/oxygenation . . . .[and] must not be provided to patients with uncontrolled diabetes, vasculitis, rheumatoid arthritis or rheumatoid ulcers, radiation and/or chemotherapy within one month or immediately preceding application, on-going use of high dose corticosteroids or immunosuppressants . . .
I thought to myself, “Where have I seen that list before?” Oh yes, I know where that list came from! It came from the exclusion criteria of the Apligraf trial that I had just finished. Here are only a few of the exclusion critera for that trial performed 26 years ago. The patient could have NO: tendon, muscle, joint capsule exposed, venous stasis for the diabetic foot ulcer trial (or diabetes for the VLU trial), alcohol/drug abuse, anticoagulant treatment, cellulitis or local wound infection, cancer or recent cancer treatment, collagen vascular disease/connective tissue disease, rheumatoid arthritis/autoimmune disease, any type, corticosteroid treatment for any reason, gastrointestinal disease of any kind /any liver disease/Hepatitis, renal impairement/ESRD/renal dialysis/renal transplant, HbA1c > 8-10, nutritional impairment, osteomyelitis, peripheral arterial disease/ischemia. In other words, the Medicare coverage policy exclusions had been taken directly from the exclusions of the clinical trial.
Nothing has changed in 25 years. The proposed LCD(s) for CTPs/skin subs in diabetic foot ulcers (DFUs) and Venous Leg Ulcers (VLUs) describes the wounds for which CTPs are “not medically reasonable and necessary” this way:
Not medically necessary in the presence of:
- Uncontrolled diabetes
- Active infection
- Active vasculitis
- Ischemia
- Placement on a necrotic wound bed
Wounds also have to be “non-infected” and undergo “debridement to a clean granular base.”
The truth is, the proposed LCD(s) are actually less restrictive than the one I was dealing with more than 20 years ago. At least the proposed LCD does not say you can’t use them in patients with any autoimmune disease or taking steroids. It does say that there needs to be “attention to” a list of medical problems including tobacco use, ischemia and nutrition, but doesn’t explain exactly what criteria have to be met to say those have been “attended to”.
Everyone is focused on the fact that the proposed LCD restricts CTP use to a short list of products. That’s not what really restricts the use of CTPs/skin subs. What restricts their use is the fact that the wound has to be practically healed for them to be covered. Most of us would say that if there is a clean, well granulated wound in a relatively healthy patient for whom all other problems are corrected, the DFU or VLU should be able to heal without a CTP.
How are CTPs used in the real world? Data from the US Wound Registry (USWR) shows that CTPs are routinely used for severe ulcers, including those over muscle, tendon and bone. In fact, only 20% of the time are they used in wounds that are documented to have 100% granulation and 0% necrotic tissue. A higher percentage are used on wounds with >50% granulation, but a lot on wounds with no granulation tissue documented. And the reason that there is necrotic material in the wound bed is often because the wounds are ischemic or inflammatory and should not be debrided.
How did we get into this mess? We got into this mess because prospective randomized clinical trials (RCTs) required by the FDA to establish efficacy are “non-generalizable” to the general population, and that is how coverage policy is written. Dr. Marissa Carter and I published the first paper on this in 2009 in which we showed that 75% of patients would have been excluded from every prospective trial that had been done to that date. “Estimating the Applicability of Wound-care Randomized Controlled Trials to General Wound Care Populations by Estimating the Percentage of Individuals Excluded from a Typical Wound Care Population in Such Trials:” Marissa J. Carter, Caroline E. Fife, David Walker, Brett Thomson, Advances in Skin and Wound Care,2009, 22: 316-24.. Patients live in the real world. For them, we need effectiveness studies.
That’s why I stopped doing RCTs more than 20 years ago and decided to spend my life focused on what seemed an impossible idea at the time – developing a registry for real world data (RWD) to enable effectiveness research. That is how the US Wound Registry (USWR) came into being in 2007. The goal of studying effectiveness in the general population is to “find what works” in the real world, and then get payers to cover it.
The restrictions of the proposed LCD are not going to be fixed by bunch of prospective RCTs with restrictive enrollment criteria on shallow, granulated wounds. Those trials probably will get a new product on the coverage list – so I understand why there’s a rush to do them. However, those RCTs – at least the way we do them now, won’t get CTPs covered for the patients who most need them. They only way to get CTPs covered for the typical patient is to use real world evidence to determine whether and in which patients they are effective. I do not know if manufacturers will invest in that research, or whether payers will listen to it. I do know that we don’t have a choice if we want to remove any of these restrictions (or get new indications added such as for pressure ulcers/injuries). I do know that it’s being done in other fields and it’s working there.
In the meantime, physicians are being audited for their use of CTPs and may pay money back to Medicare because they were using CTPs as a “Hail Mary” pass on difficult patients. What physicians need to know is that using CTPs/skin substitutes on those “difficult cases” has NEVER been covered by Medicare, and that will not change if the proposed LCD(s) are finalized. We should stop the public outcry about how “vulnerable patients” with “the most serious limb threatening” wounds will lose access to these products. Those patients have never had Medicare coverage for CTPs.
–Caroline

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.