United Healthcare has determined that amniotic tissue products are unproven and will therefore not be reimbursed because of “insufficient clinical evidence of safety and/or efficacy.” As I have mentioned before, the Medicare Advantage plans in my area have been following the “Nancy Reagan” approach to any cellular and/or tissue based product (CTP) that was cleared through the 510k pathway – which is, “Just say no.” In my experience, the Medicare Advantage plans are only covering Apligraf and Dermagraft, and since they are administered by private insurers like United Healthcare, the recent announcement by them is not surprising. However, the real issue is not whether we can use cellular products other than “the original two.” The question is whether we can use ANY cellular products to treat chronic ulcers in real patients.
This storm cloud has been forming for decades. I was one of many co-investigators in the original RCT for Apligraf in the 1990’s. I was new to wound care and excited about the possibilities of cellular therapy. However, I was unable to enroll more than a handful of patients from the wound center because my “real world” patients were all too sick and had too many of the exclusion criteria. Since that trial was before the advent of “social media,” we had to advertise for study subjects in the newspaper and on the radio. It was years before I understood that my regional Medicare Administrative Carrier (MAC) was crafting coverage policy based on those RCT exclusion criteria, which meant that these products would not be covered by Medicare if I used them on patients with the co-morbid diseases common in my practice. If you don’t believe me about how different real patients are from those enrolled in RCTs, take a look at two tables from a paper we published a few months ago. We compared the real patients seen in 6 clinics with the subjects those same clinics enrolled in 5 cellular product RCTs. There was virtually no overlap between the real world patients and the subjects enrolled in CTP clinical trials.


That’s why nearly 2 decades ago I stopped doing RCTs (even though I was still in an academic medical center with a faculty appointment), and started trying to find a way forward for Real World Data – even though I couldn’t get federal funding. But times have changed. This month Dr. Jacqueline Corrigan-Curay and colleagues at the FDA Center for Drug Evaluation and Research have a Viewpoint article in JAMA about “Real-World Evidence and Real-World Data for Evaluating Drug Safety and Effectiveness.” Under the 21st Century Cures Act, the Food and Drug Administration is tasked with developing a program to evaluate the use of Real World Evidence (RWE) to: 1) support approval of new indications for approved drugs and/or 2) satisfy post-approval study requirements.
Real World Evidence is the clinical evidence regarding the benefits or risks of a medical product obtained from the analysis of Real World Data (RWD). The biggest barrier to obtaining useful RWD from electronic health records (EHRs) that that generally EHRs lack sufficient structure (outside of diagnosis codes and medications, etc.) to glean anything terribly useful. But, wound care is a very iterative process, and two decades ago we structured nearly every bit of it. Then we harnessed quality measures to standardize “usual and customary care,” and leveraged the Quality Payment Program requirements for registry participation to get 130+ centers on board with pooling their data.
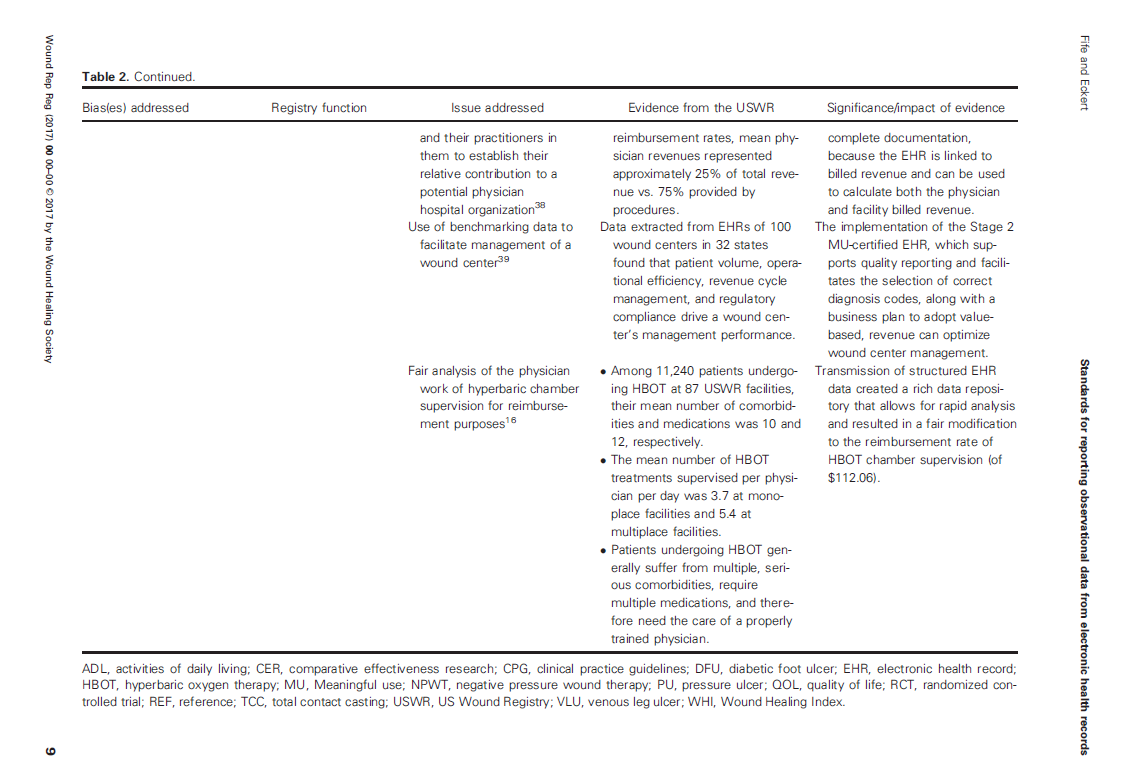
To have data that the FDA will accept, there are many sources of bias that have to be accounted for. We established some of those standards in the paper, “Harnessing electronic healthcare data for wound care research: Standards for reporting observational registry data obtained directly from electronic health records,” calling the approach the “ABCs” or “Analysis of Bias Criteria” system. We hoped these ABCs would establish standards for studies using EHR data like the “CONSORT” criteria do for prospective trials.
If you are going to use registry data from EHRs for establishing real world effectiveness, then the Methods section of a paper has to tell us who entered the EHR data, when it was entered, whether it was the legal medical record or some ancillary system, how the EHR data was transmitted to the registry, whether it could be changed at any point, how it was secured, when it was de-identified, whether it included all the patients and wounds available or only some of them, and what percent of patients had unknown outcomes, to name only a few. EHR data should include medications and co-morbid conditions to enable risk stratification and cohort matching – otherwise, what is the point of using EHR data?
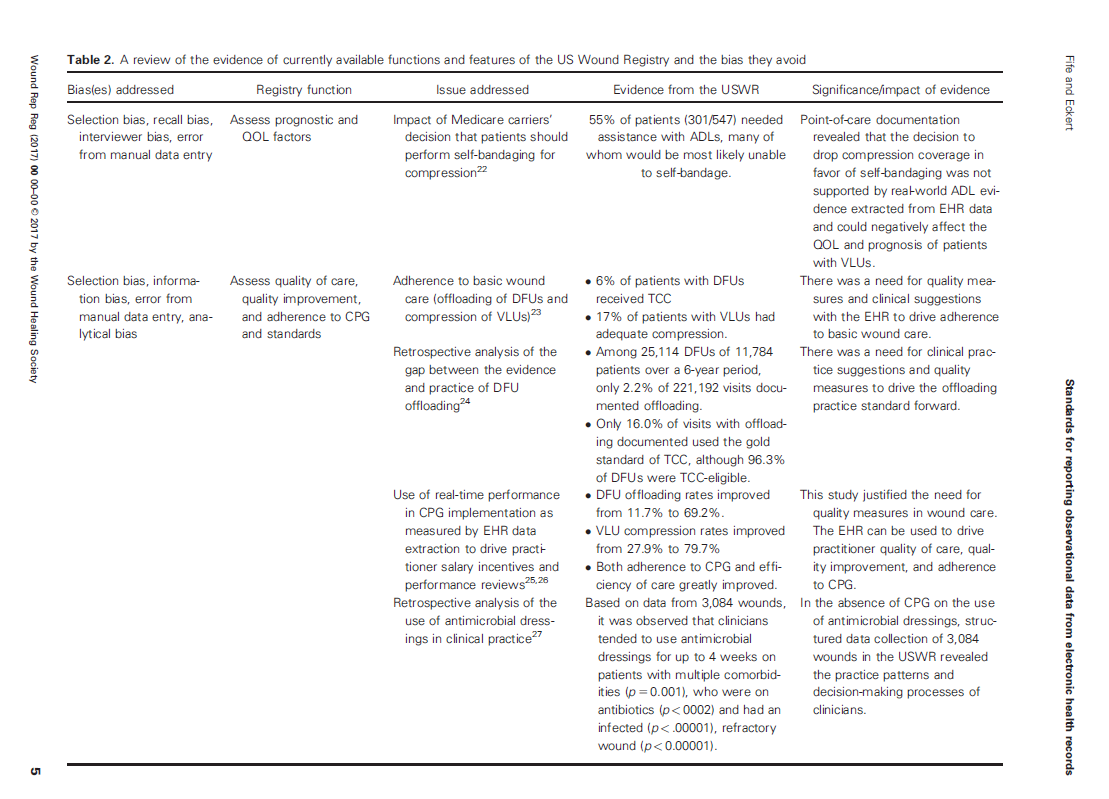
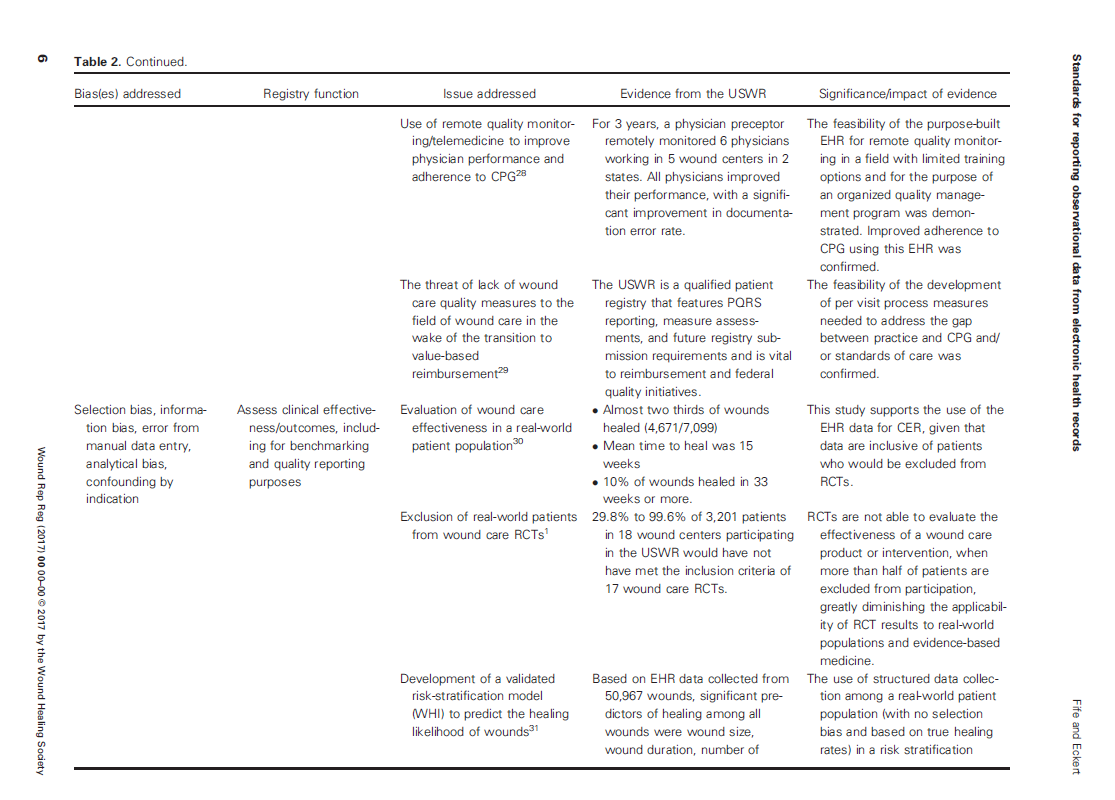
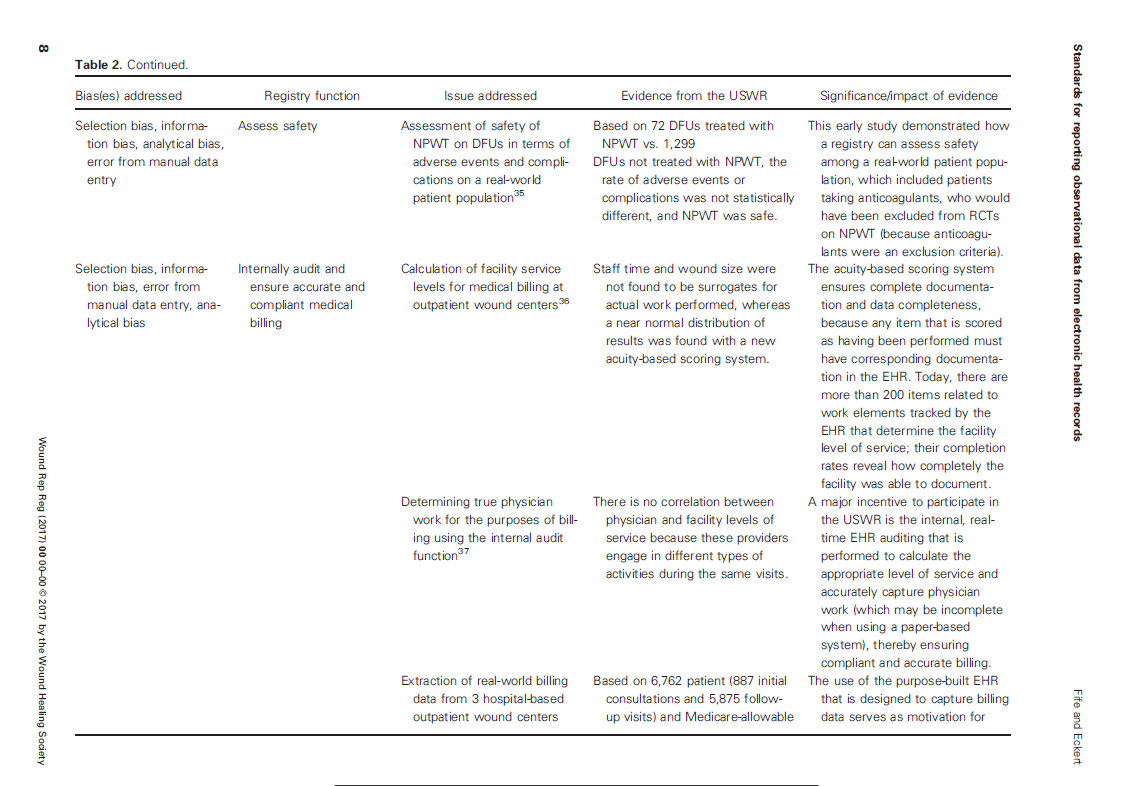
The various sources of bias and how the US Wound Registry handles them are listed in Table 2 which I have provided below:
Getting a “direct from EHR” registry to the point that data from it might be taken seriously by the FDA has been a massive undertaking two decades in the making. Why have we done it? We did it because as Medicare and other payers begin to ration care, it was inevitable that they would stop covering advanced therapeutics to real world patients making the argument that the effectiveness of these interventions has not been proven. This is not just about whether the “other” CTPs can be used, but whether we can use ANY of them in real world patients.
It’s time to demonstrate to the FDA and CMS what real wound care patients look like – through their co-morbid conditions, their medications, how many wounds they have, how long they stay in service, and their honest outcomes. That sets the stage for a discussion about why advanced therapeutics are needed, and effectiveness in the real world looks like. If we don’t do that, we won’t be able to use ANY of them in real world patients.

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.