The Wound Care Evidence Summit will take place in Washington, D.C. April 1-2. If you are involved in this area, you need to register now because spots are limited. The meeting will convene commercial and government payer medical directors, the FDA, NIH senior staff, wound care researchers, manufacturers, and policymakers. Here’s a link to the agenda.
I thought I’d explore some of the issues that have troubled me since I got into this field.
My epiphany came in about 1999 around the case of a 72 year old Latin American woman with rheumatoid arthritis on prednisone and methotrexate who had poorly controlled diabetes. She had a 5-month history of an enlarging leg ulcer due to minor trauma (probably an inflammatory ulcer, but the biopsy was negative). I had been using “the VAC” for about 2 years and had been one of the several investigators on the Apligraf clinical trial. I used both of those relatively new modalities to heal her. The photos below are not impressive today, but they were (at least to me) in 1999. The photo quality is poor because they were originally polaroids. The problem is that I realized after the fact I had committed Medicare Fraud.
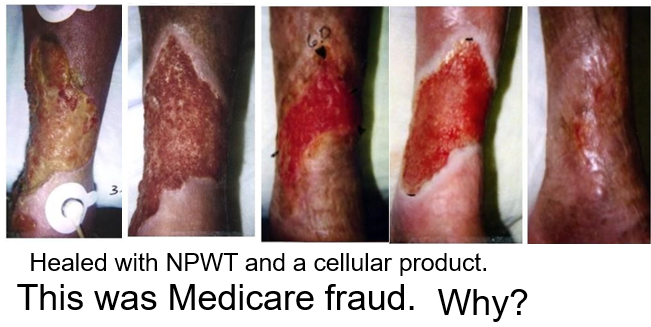
I was running the only wound center in the vast Texas Medical Center in Houston. I thought I was doing clinical trials with these promising “biologics” so that patients like this woman could be helped. My clinic was incredibly busy, but I had to advertise in the newspaper for subjects to enroll in the trial. Why?
Here’s a partial list of the exclusion criteria for every cellular product trial done over the decade between 1996 and 2006 when we first started on this journey to harness healing at the cellular level:
- For DFU studies, no ulcers > Wagner Grade II
- Diabetes as a co-morbid condition for any study other than DFU
- Venous stasis except in VSU trials
- Alcohol/drug abuse
- Anticoagulant treatment
- Cellulitis or local wound infection
- Cancer or recent cancer treatment
- Collagen vascular disease/connective tissue disease
- Rheumatoid arthritis/autoimmune disease, any type
- Scleroderma/lupus, any autoimmune disease
- Charcot foot changes in DFU
- Corticosteroid treatment any reason
- Deep venous thrombosis/pulmonary embolus
- Gastrointestinal disease of any kind /any Liver disease/Hepatitis
- Renal impairment/ESRD/Renal dialysis/Renal transplant
- Any organ transplant
- In diabetics, HbA1c > 8-10
- Nutritional impairment/Albumin < 3.0 mg/dl
- Osteomyelitis
- Peripheral arterial disease
The above list is the reason that my Medicare Administrative Carrier (MAC) – which at the time was Trailblazer, listed the following criteria for the use of “skin substitutes” in its Local Coverage Determination (LCD):
“. . .must not be provided to patients with: uncontrolled diabetes, vasculitis, rheumatoid arthritis ongoing use of corticosteroids or immunosuppressants. . . .”
The exclusion criteria of the clinical trials was enshrined in coverage criteria. I was performing randomized, controlled trials in order to bring to market products that I would not be able to use in the very patients who most needed them. I was on the Institutional Review Board (IRB) of the University of Texas, Houston and I knew that the RCTs I was doing were not unethical. However, I decided they were just WRONG. I couldn’t keep doing them. By 2002, I realized I couldn’t participate in an RCT that excluded the patients who were most in need of the product. It didn’t make any difference what the smart people in the room said about beta error and other details of trial design. It was still wrong.
In 2008, I asked Marissa Carter to help me analyze a decade of trials using the registry data that is now called the U.S. Wound Registry (USWR). We found that among 8,611 wound center out-patients, > 50% would have been excluded from 15/17 major wound product RCTs. In fact, 88% of real world patients would have been excluded from prospective trials at the “first pass,” just on the basis of their comorbid conditions and medications. More worrisome, 3 out of 4 major trials that brought new products to market had enrolled subjects healthier than the “man on the street,” based on disease prevalence. We were doing trials on wounds likely to heal without them, and among the healthiest people we could find who still had a wound.
In 2008, our registry data showed that the average patient was 60 years old, had a wound that had been present for 6 months when they were first seen, and had 6 serious comorbid conditions: 16% had coronary artery disease, 8.4% were on prednisone, 5% had renal failure or a transplant, and 26% of wounds that were not specifically diabetic foot ulcers were in patients who had diabetes. I can tell you, now that the USWR has hundreds of thousands of patients, the prevalence of those diseases is even higher than we found in 2008.
That’s why by 2005, what became the US Wound Registry was launched. We took it for its first spin in 2007 to understand the safety of “the VAC” among patients on anticoagulants. I stopped doing sponsored RCTs because they were non-generalizable and physicians by and large did not understand that when they used what we now call “Cellular and/or Tissue Based Products” (CTPs) outside of the coverage policy in their respective LCDs, they had legally committed Medicare fraud and could have to pay the money back. Audits of CTP use have begun. Manufacturers keep performing clinically irrelevant RCTs, in part because they have to meet specific FDA requirements, and payers keep crafting coverage policies that exclude the patients who most need the products, using the irrefutable logic that the effectiveness of the products is not known among sick patients. Kafka himself could not write a better story than currently exists in wound healing research.
“Estimating the Applicability of Wound-care Randomized Controlled Trials to General Wound Care Populations by Estimating the Percentage of Individuals Excluded from a Typical Wound Care Population in Such Trials:” Marissa J. Carter, Caroline E. Fife, David Walker, Brett Thomson, Advances in Skin and Wound Care,2009, 22: 316-24.

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.




https://www.globenewswire.com/news-release/2016/10/31/884936/0/en/Peer-reviewed-Publication-on-the-use-of-Grafix-Prime-for-Outpatient-Management-of-Complex-Wounds-with-Exposed-Bone-Tendon-Muscle-Joint-and-Hardware.html
Dr. Fife,
Here is the type of study you might be looking for. I know this is in-line with our patient population and limb salvage needs. We needed grafts for hard to heal wounds and not easy wounds (those heal with debridement and clean moist dressings). This study helps us prove medical necessity in patients without other options. Have a great day!
Sincerely,
Sharon Hannan
You are right! This is what we need to be doing! Now all we need is for the FDA indications and the payer coverage criteria to align for these patients. As long as private payer coverage criteria and MAC LCDs say they don’t cover a product (at all or in these particular wounds-patients), the problem still isn’t FIXED. Then the product pricing has to work under package pricing (and now bundled payments)- for use with products BIG enough to cover the surface area of real world wounds. Then it’s fixed! But hats off to any company doing generalizable trials!