Racing to Find Better Treatments for COVID-19 Pneumonia
The COVID-19 pandemic, and the severity with which COVID-19 pneumonia has affected some patients, is spurring many clinical trials. Many (if not most) of these trials are evaluating the effectiveness of drugs that are currently FDA-approved in the treatment of other conditions. There’s nothing unethical about treating a COVID-19 patient with a drug that is FDA approved for a different condition. It’s called “off-label” use, and we do that nearly every day in clinical practice. However, deciding to give the drug to a series of patients to evaluate what happens to them is called “research” and requires an IRB-approved protocol and a research consent. That’s all pretty straightforward.
Should we keep promising preliminary results confidential?
There IS debate about whether anecdotal or preliminary information about an off-label drug can or should be shared publicly. A treatment can appear to be useful in a few patients – based on lab values or some intermediate endpoint – but then show no benefit in the final analysis. If encouraging preliminary information is shared openly, expectations are raised, and others might try the off-label drug with negative consequences. Then there is the risk of jeopardizing publication of the data by making some of it public ahead of time, or the risk of a HIPAA violation if a small group of patients/subjects have received it and it might be possible to figure out exactly who they were.
On the other hand, COVID-19 has presented us with challenges we’ve never seen before. If an available, common drug appears to be helping seriously ill patients to avoid intubation and be discharged earlier, most of us don’t want to wait a year or more for the completion of a trial to find that out. Lives are at stake, and the price tag for seriously ill patients is staggering. Our colleagues are getting sick, our family members are getting sick. If there’s a game-changing treatment, most of us would like to know.
Most people would say we can’t tell you what Drug X actually is
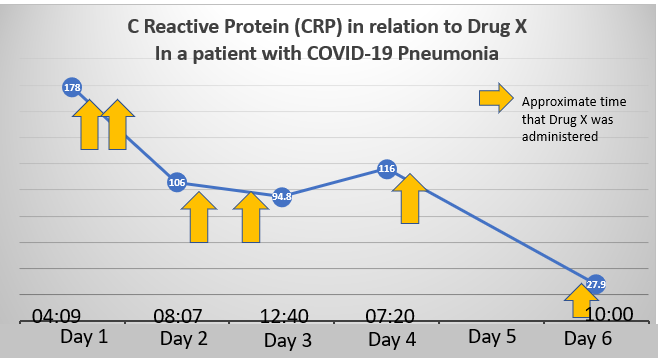
Above is a graph of the C-reactive protein (CRP) level in a patient admitted with COVID-19 pneumonia. In addition to his significant respiratory complaints, he had severe body aches and gastrointestinal symptoms. He received a currently available drug, for which COVID-19 represents “off-label” use. He did not require intubation and was discharged from the hospital on the date of the last blood draw, 5 days after admission. Most very sick patients treated with this drug have avoided intubation. The patients are enrolled in a small trial that will NOT be considered definitive because it’s not prospectively randomized. As is often the case when new things are tried, we first evaluate whether it even has promise by treating a small group of subjects (enrolled in an IRB-approved trial), and then evaluating what happens to them. So, no matter when the data are released, this small trial will not convince anyone that the treatment worked. It can tell us whether this treatment should be pursued. And before anyone gets too critical, the trial is unfunded. Lots of exhausted clinicians are donating their time to do these projects.
What should we do? The graph above proves nothing. But the early open label trial results have been very promising. Can we, or should we, talk about the preliminary results of Drug X?

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.



People has promoted drugs on far less data. However, it would be nice to see more than one and see general trends.
When people are dying, and it appears we have a solution from small studies done at several locations follows the legal point of being in “ best medical opinion” where the risk clearly don’t outweigh the benefit of rendering aid , especially in light of FDA Approval for 13/14 conditions. This is a no brained, and IS the answer as time will tell!
Since drug X is showing efficacy in other studies, this is called a ‘body of evidence’ that needs to get to anyone in influence who can get it to the right people! Gen John Wharton should be consulted…