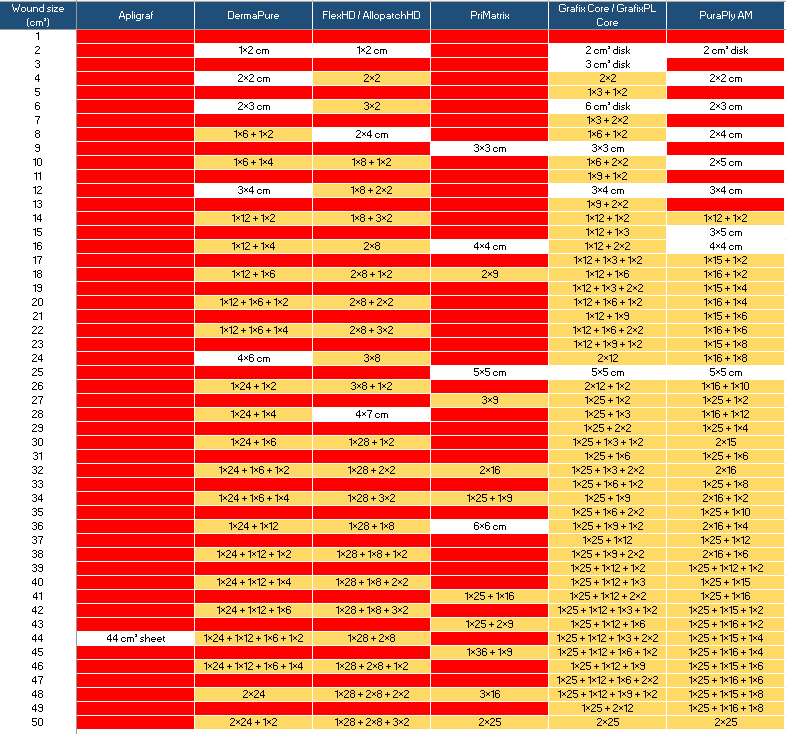
How to interpret the table: Wound sizes in cm2 are represented from 1 cm2 to 50 cm2. Each column represents a product, and the various available sizes are represented in the white fields. For example, a wound that is 2 cm2 would be treated by a product that is 1 x 2 cm2 (visible as a white field). The fields in red are wound sizes that cannot be treated by that product without wastage, based on the available size(s). The larger a product is, the harder it is to use without wastage. The gold fields represent the wound sizes that could be treated without wastage by “scrapbooking” various sizes of the product. In some cases, four different sized products would be needed to get the “no wastage” math just right.
As examples, I picked a few CTPs that were affordable as of the last quarter of 2025 (meaning the ASP was significantly under the $127/cm2 payment limit).
I am aware that some products have already announced new pricing which may make them unaffordable, but remember, this is a mental exercise. I know that I might have gotten some sizes wrong — and those of you who do not have a personal life can correct my math. While this was a theoretical exercise, the clinical impact is very real. Sadly, this is how problems of abuse get “fixed” in the Medicare system. Payment policy is not a delicate surgical instrument; it is a baseball bat.
Here are some things this little mental exercise has taught me:
- Given the incredible range of wound sizes, it is almost impossible for a practitioner to use only the exact amount of a product that would cover the wound bed – there will HAVE to be wastage.
- Manufacturers are going to have to build “wastage costs” into the price of their product.
- Minimizing wastage will take a lot of “scrapbooking” and this will be a huge time suck for clinicians.
- CTP “scrapbooking” will probably mean that practitioners can use only long shelf-life products that come in multiple sizes.
- Live/fresh products that come in one size will be nearly impossible to use –not only because fewer than 10% of all CTP applications are on wounds larger than 44 cm2, but because even very large wounds can only be treated if they are exactly a multiple of the product size (otherwise, you have to waste a portion of the second piece).
- It will be very hard to treat small wounds without losing money.
- The CMS policy of not paying for wastage is going to be a bigger barrier to skin sub use than the price decrease.
- The winner of the new CTP market will be the company that prices their product low enough to allow the amount of wastage that is INEVITABLE – and that has a lot of different sizes.
Now for some history. In 1999, after we finished the clinical trial of the first product to market (you know who I am talking about), and we started using this novel treatment in the wound center, there were no wastage codes. Remember, this was before “package pricing” so the hospital was buying what was at the time, a very expensive live product that had to be used within a couple of days. There was no way we were going to waste ANY of it. We developed a system to keep the live product in a clean and temperature controlled area and scheduled all the patients who would benefit on the same day. In a clean area outside of the examination room, we used sterile technique to cut up the product into the necessary sections and then brought the needed amount into the room to apply it. Remember, it was an entirely new treatment and we approached it like an expensive chemotherapy drug from which the necessary amount for each patient was removed from a vial. We did not waste even one cm2. Although we had no issues with this system, Hospital infection control put a stop to the practice. Infection control (and the Joint Commission) forced us to throw in the trash an expensive treatment that a patient in the next room might have desperately needed. To keep hospitals from giving up on this treatment, wastage codes had to be developed. Eventually, long shelf-life products came on the market. But even long shelf-life products are labeled “single use” so a practitioner cannot keep the remainder even for use in a specific patient. Again we are forced to throw away the remainder of a product with a long shelf life that could be used on that same patient during their next visit. The folks who abused the wastage codes got us into this mess. Either new packaging will have to be developed, or manufacturers will have to “bake” wastage into the product price.
–Caroline

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.