
by Caroline Fife, M.D. | Oct 29, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
On October 8th, Med Page Today published an opinion piece by physicians Thomas Lally and Michael Millie about the increasing use of skin substitutes in chronic wounds. Their independent medical group cares for more than 100,000 seniors across 17 states, which...
by Caroline Fife, M.D. | Oct 28, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
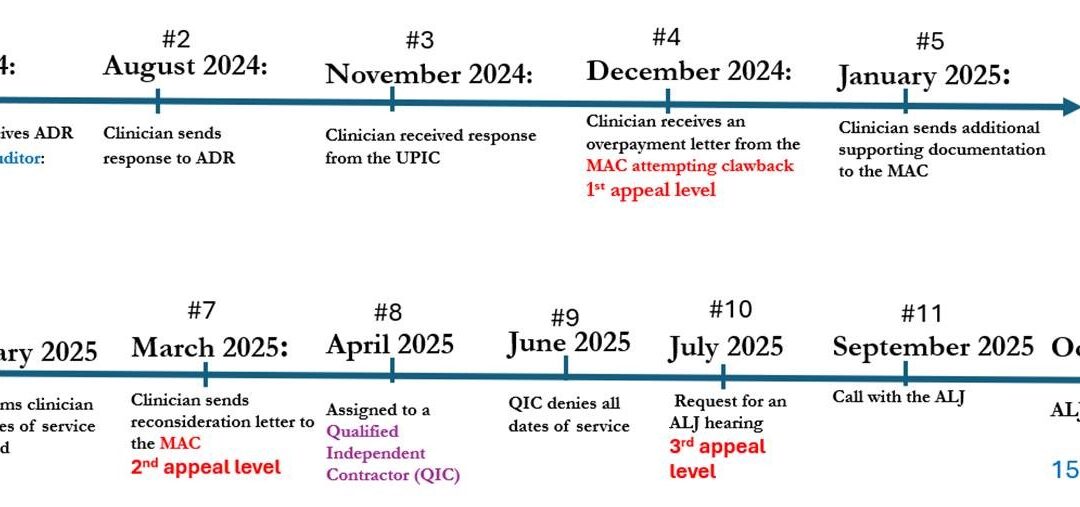
Guest Blog by Michael J. Crouch, CPC, CPMA, CHT-ADMIN Dr. Fife – In March, I provided feedback on a skin substitute graft (SSG) audit in which I was involved that was similar to the one Martha Kelso described. The Unified Program Integrity Contractor (UPIC)...

by Caroline Fife, M.D. | Oct 16, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitute Arrests, CTP / Skin Substitutes
Alexandra Gehrke and her husband, Jeffrey King of Arizona pleaded guilty to over $1.2 billion of false and fraudulent Medicare and other insurance claims for, “expensive, medically unnecessary wound grafts that were applied to elderly and terminally ill patients.” The...

by Caroline Fife, M.D. | Oct 13, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitute Arrests, CTP / Skin Substitutes
Eastern District of California | Fresno County Podiatrist and Sales Representative Plead Guilty to Conspiracy to Submit False Claims Related to Skin Grafts | United States Department of Justice US Attorney Eric Grant has announced that Felipe Ruiz, a podiatrist at...

by Caroline Fife, M.D. | Sep 26, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
For the past 3 years I have been watching the “skin substitute” industry and asking what I thought were simple questions about pricing, payments, and the legality of things like “selling the spread.” I have been worried about the possibility that unwary...
by Caroline Fife, M.D. | Sep 15, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
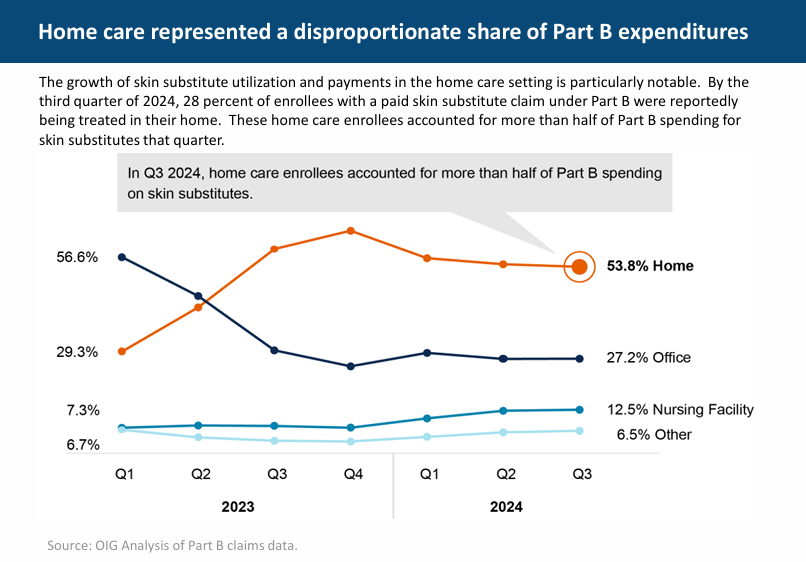
The Office of the Inspector General (OIG) has just released a report on Medicare claims for “Skin Substitutes”. OIG analyzed Medicare Part B claims data, Medicare Advantage claims data, and manufacturer-reported sales data for skin substitutes in 2023 and 2024....
by Caroline Fife, M.D. | Sep 12, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
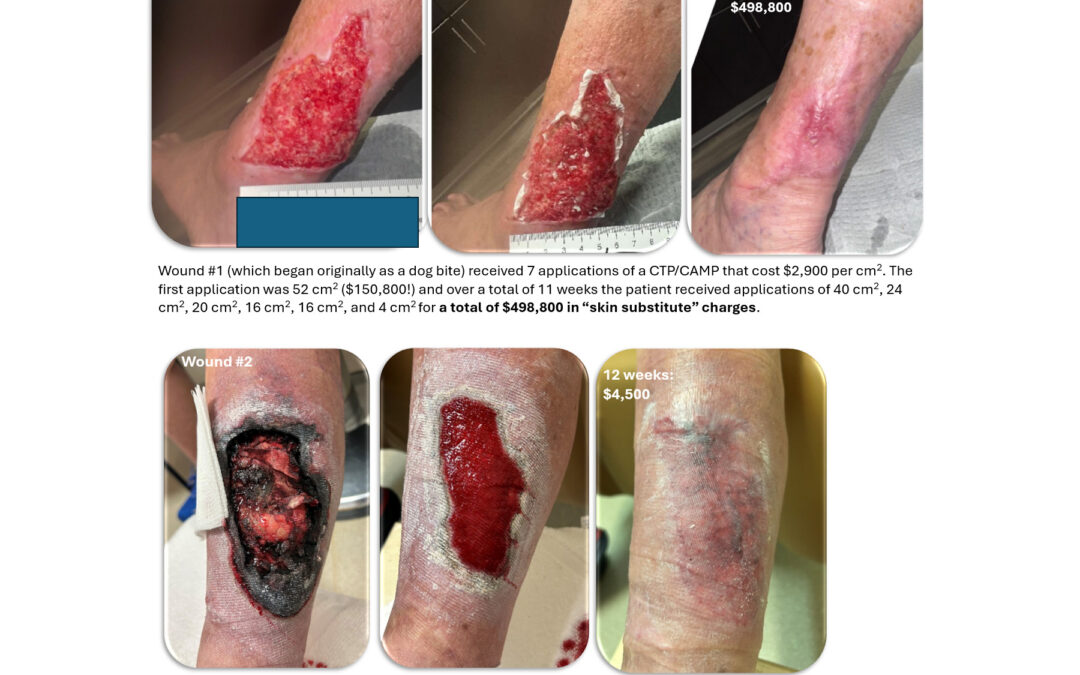
Dr. Fife, I wanted to provide some follow up on the “Tale of Two Wounds”. Eventually, Wound #1 cost Medicare (and the taxpayer) almost half a million dollars whereas Wound #2 cost less than 1% of that. And since the patient is responsible for 20% of those Medicare...

by Caroline Fife, M.D. | Sep 8, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
Dr. Fife, Your articles on skin subs are so helpful. An old coworker of mine called to ask me about the wound care Nurse Practitioner jobs she had seen advertised about applying skin subs. The company did a video interview with her, telling her how much money she...

by Caroline Fife, M.D. | Aug 20, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitute Arrests, CTP / Skin Substitutes
I recently reviewed with interest the press releases and case summaries issued by the Department of Justice (DOJ) related to individuals charged in the 2025 National Health Care Fraud Takedown for their involvement in the “skin substitutes” space.““` With...

by Caroline Fife, M.D. | Aug 12, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
Attorney Knicole Emanuel’s most recent blog post is about Medicare audits focused on the use of “skin substitutes” by Recovery Audit Contractors (RACs) and Unified Program Integrity Contractors (UPICs). The proposed skin substitute LCD(s) may be on hold, but that has...












