
by Caroline Fife, M.D. | Jun 2, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
I had a great time speaking at the WOCN conference in Orlando! I was asked to provide an update on “Skin Substitutes.” I will give you a few highlights from the talk. I reviewed the reasons we need skin substitutes, such as the huge percentage of patients suffering...

by Caroline Fife, M.D. | Apr 28, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes, The Patient's Voice
I just got this letter by email from a physician emphasizing (in addition to the ethical issues) the need for clinical studies of Cellular Tissue Products / Skin Substitutes on pressure ulcers. Thoughts anyone? –Caroline Dear Dr. Fife, I am writing to express a...

by Caroline Fife, M.D. | Apr 23, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
Hi Dr. Fife, Here’s my two cents as someone who has lived this mess for a long time now. The root cause of this issue is the CMS payment structure. CMS has known about this problem this for years. Every year they talked about fixing it and every year they chose...
by Caroline Fife, M.D. | Apr 16, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
SmartTRAK is reporting that in the 4th quarter of 2024 (Q424) the “skin substitute” market reached almost $2 billion, an increase of 25% compared to the 4th quarter of 2023 (Q423). Human amniotic products represent the majority of the US market at 76.3%, up from 72.1%...

by Caroline Fife, M.D. | Apr 14, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
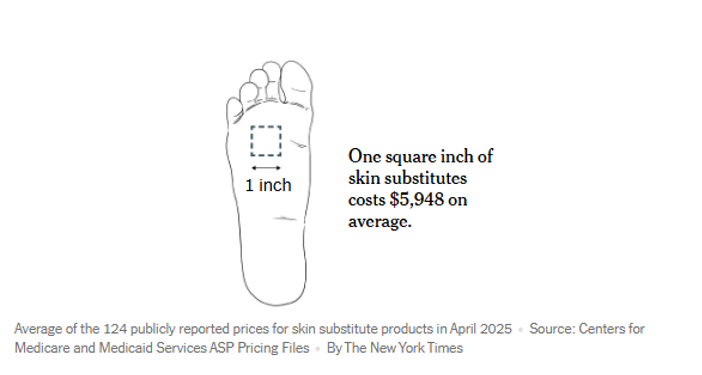
I didn’t write the New York Times article about “skin substitutes” It was written by Sarah Kliff and Katie Thomas. You can send them your comments at nytimes.com/tips. Jeanne Pinder at Clear Health Costs also has a new article: “Wound Care Regulations Delayed...
by Caroline Fife, M.D. | Apr 11, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
Check out the article in the New York Times about “skin substitutes.” Medicare Bleeds Billions on Pricey Bandages, and Doctors Get a Cut – The New York Times “Medicare spending on ‘skin substitutes’ made of dried placenta has soared as doctors pocket...
by Caroline Fife, M.D. | Apr 11, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
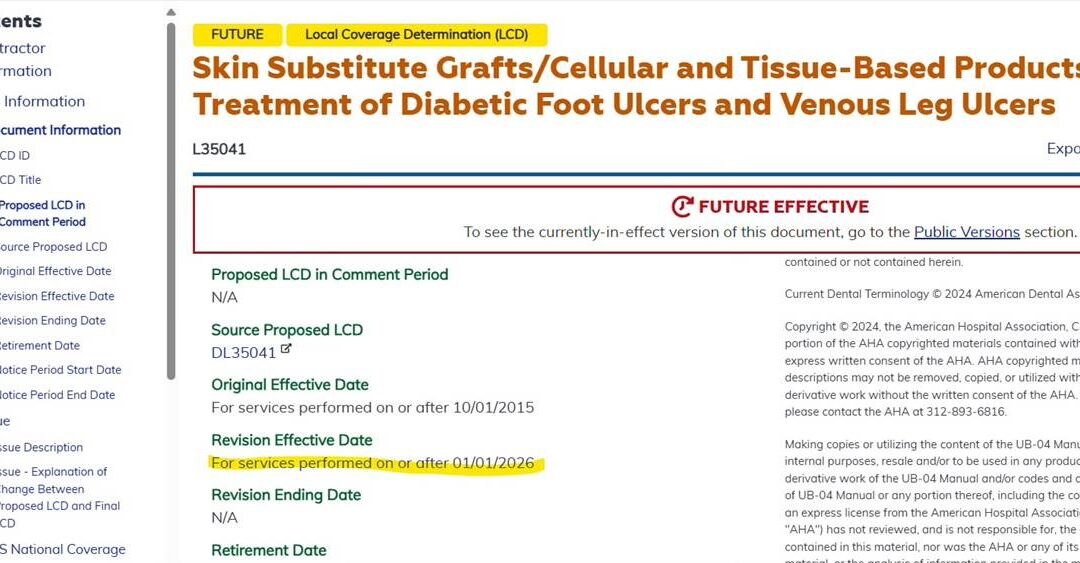
LCDs appear to be delayed until 1/1/26 based on MAC websites changing the revision effective date. More news as I get it. There’s now an amniotic that is over $5,000 per cm2. I wonder how high the prices will go by next January? CMS Statement on Local Coverage...

by Caroline Fife, M.D. | Apr 9, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
An opinion piece has been published in RealClear Health about the upcoming launch of the “skin substitute” LCD(s), authored by the president of Consumer Action for a Strong Economy (CASE). (RealClear Health is part of RealClear Politics). The article...

by Caroline Fife, M.D. | Apr 8, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
Here’s another guest blog from Michael Crouch who is a professional coder and medical auditor, detailing the documentation demands of the LCDs. It’s true that we don’t know if the LCDs will be implemented on April 13th. However, with or without the implementation...

by Caroline Fife, M.D. | Mar 28, 2025 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
Welcome to the Circus: The State of Healthcare in America | medicaidlaw-nc Attorney Knicole Emanuel has another blog post (yes, I am a big fan of her blog) about Medicare audits. She describes “compliance” as a “Kafkaesque masterpiece of government regulation.” The...












