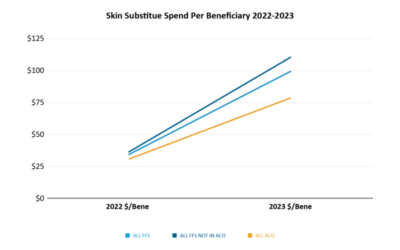
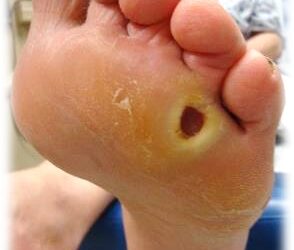
CTP / Skin Substitute Arrests
Arizona Couple Sentenced to Prison Over “Skin Substitute” Fraud – Judge Called Them “Criminally Greedy”
Fresno County Podiatrist and “Skin Substitute” Sales Representative Plead Guilty to False Claims
The Devil is in the (Contractual) Details: Avoiding Potential Kickbacks in the World of Skin Substitutes (Guest Blog by David Traskey)
More Info on the Individuals Indicted by the DOJ Around the Use of “Skin Substitutes”
BREAKING NEWS in the DOJ Press Conference! More Skin Substitute / Cellular Tissue Product (CTP) Fraud Indictments
Houston Podiatrist and Business Partner Charged with $45 Million in Healthcare Fraud Related to “Skin Substitutes”
Federal Investigators Probe Wound Care Industry – ClearHealthCosts
BREAKING NEWS: Wound Care Company Owners Plead Guilty to $1.2 B in Fraudulent Claims for Amniotic Products and Face Prison
How Much About Access to Care With “Skin Substitutes” is Around Cost (and Will the LCDs Actually Make That Better for Patients)?
Slide Deck for the December 20, 2024 CGS Education Webinar on the Soon to Be Implemented “Skin Substitute” LCD
The US Cellular Tissue Product (CTP) / Skin Substitute Market Reached $1.75 Billion in the Third Quarter of 2023, Based on SmartTrak Analysis
Compared to the Third Quarter, the Cellular Tissue Product (CTP) / Skin Substitute Market Grew 64% in the Fourth Quarter of 2024
The Accountable Care Organizations are Watching…
Get that Skin Substitute “Clawback Insurance!”
“I’ve Consistently Been Told the Answer is Yes… At Least, I Hope it’s Yes — Otherwise, We’re All in Trouble.”
Why Aren’t Patients Up in Arms About Huge Copays for Cellular Tissue Products (CTPs) / Skin Substitutes?
According to Medicare Data Analyzed by SmartTRAK, Recent DOJ Prosecutions in the Cellular Tissue Product / Skin Substitute Market are the “Tip of the Iceberg”
Two Nurse Practitioners Charged in Connection with APX Scheme with Fraudulent Application of Amniotic Products
Wound Care Company Owners Arrested, Accused of Accepting More Than $330M in Kickbacks Relating to Medicare Charges for Amniotic Products
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.