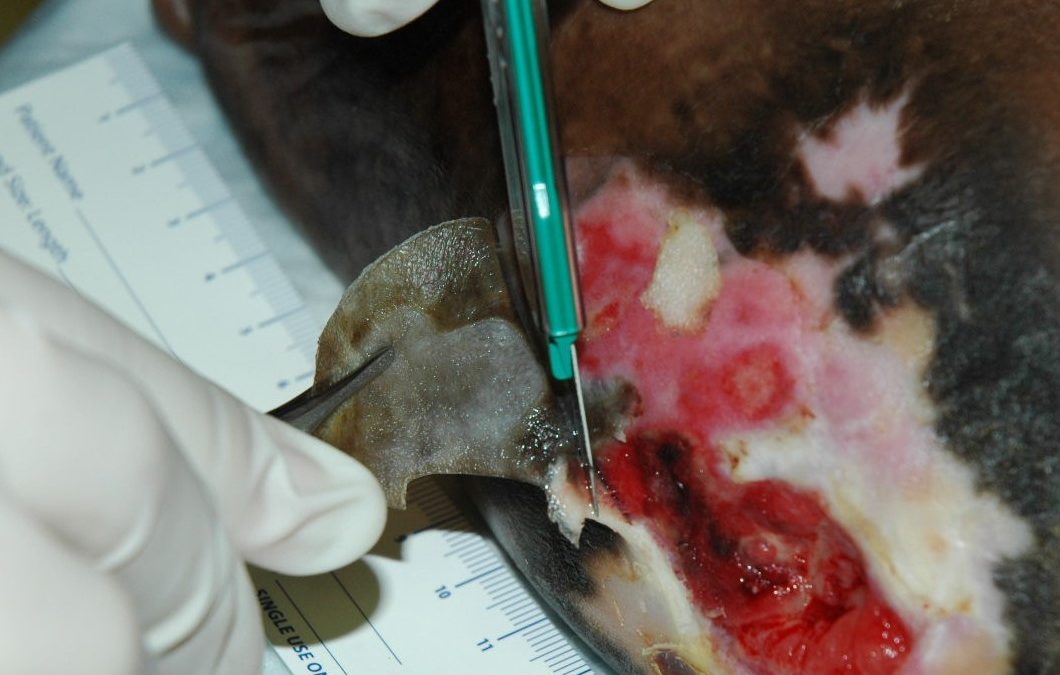
I’ve been blogging about debridement as it relates to the Novitas Local Coverage Determination (LCD) on Wound care issued in final form just recently. (You can find a pdf version of the LCD here).
The Novitas LCD states that debridement services can be continued beyond a total of 8 subcutaneous debridments and 5 at deeper levels, “only when medical necessity continues to be met and there is documented evidence of clear benefit from the debridements already provided.”
The LCD specifies in exhaustive detail what sort of documentation is needed to satisfy medical necessity. In fact, the LCD has a lot of language making it clear that Medicare coverage for all wound care (not just debridement) is contingent upon documentation that satisfies their requirements.
You should read the entire LCD, but I thought I would point out a few things that jumped out at me. I am just the messenger. In a previous blog I discussed the way in which engagement by the Alliance of Wound Care Stakeholders avoided an even more draconian Novitas LCD and I reviewed the events over the past decade that got us here. My goal is to point out some language in the LCD you might not have noticed, and that might cause you to make some changes in your documentation. I have put verbatim quotes in quotation marks, but the bold type is mine. This is going to take a lot of engagement with your EHR vendor.
Documentation at EACH Physician Visit
“The patient’s medical record must contain clearly documented evidence of the progress of the wound’s response to treatment at each physician visit. This documentation must include, at a minimum:
- Current wound volume (surface dimensions and depth).
- Presence (and extent of) or absence of obvious signs of infection.
- Presence (and extent of) or absence of necrotic, devitalized, or non-viable tissue.
- Other material in the wound that is expected to inhibit healing or promote adjacent tissue breakdown.”
As an FYI, note that they specify “physician” visit. Other advanced practitioners count, of course (e.g. NPs, DPMs, etc.). However, if you are in a facility that still has not gotten the memo that the hospital based outpatient clinic can’t see patients unless the advanced practitioner is physically present in the facility, please bring your center into compliance with this requirement because the OIG intends to keep auditing this.
What ISN’T Debridement?
“Removal of non-tissue integrated fibrin exudates, crusts, or other materials from a wound without removal of tissue does not meet the definition of any debridement code and may not be reported as such.”
Here’s a list of services that the LCD says are NOT wound debridement services:
- Washing bacterial or fungal debris from lesions.
- Removal of secretions and coagulation serum from normal skin surrounding an ulcer
- Paring or cutting of corns or non-plantar calluses
- Incision and drainage of abscess and trimming mycotic nails
The Big 10 in your Debridement Note
The procedure note for the debridement service should include the following:
- Medical diagnosis.
- Indication(s) and medical necessity for the debridement.
- Type of anesthesia used, if and when used.
- Wound characteristics such as
- Diameter
- Depth
- Undermining or tunneling
- Color
- Presence of exudates or necrotic tissue.
- Level/depth of tissue debrided and a description of the types(s) of tissue involved and the tissue(s) removed.
- Vascular status infection or evidence of reduced circulation.
- Narrative of the procedure to include the instruments used. When debridements are reported, the debridement procedure notes must demonstrate tissue removal (i.e., skin, full or partial thickness; subcutaneous tissue; muscle and/or bone), the method used to debride (i.e., hydrostatic, sharp, abrasion, etc.) and the character of the wound (including dimensions, description of necrotic material present, description of tissue removed, degree of epithelialization, etc.) before and after debridement.
- Patient specific goals and/or response to treatment.
- Immediate post-op care and follow-up instructions.
- The presence or absence of necrotic, devitalized, fibrotic, or other tissue or foreign matter must be documented in the medical record when wound debridement is performed.
You Need a Plan of Care and Goals of Therapy
“Debridements are best provided under an individualized plan of care.”
“The medical record must include a plan of care containing treatment goals and physician follow-up.”
“The record must document complicating factors for wound healing as well as measures taken to control complicating factors when debridement is part of the plan.”
“The treatment plan for patients requiring debridements should be re-evaluated . . .”
Note that the treatment plan for patients requiring debridements must be constantly re-evaluated to ensure that a number of factors have been adequately addressed such as:
- pressure reduction
- nutritional status
- vascular insufficiency
- infection control
I wonder if anyone has noticed the recurring theme about needing a treatment plan?
The LCD also says it is necessary to “ . . .determine whether the individualized treatment goals are being met for the patient.”
That means, of course, that you must actually HAVE individualized treatment goals. The LCD says that the goal is usually healing, but could be to prevent wound progression, minimize risk of infection, or optimize patient quality of life.
Patient Quality of Life with Wounds
I need to give you fair warning. I really, really want to talk to you at the Fall SAWC in Las Vegas that is happening now. But if you use the words, “wound related quality of life” in my presence, you must be prepared to back away slowly and then turn and run away fast. The US Wound Registry paid to license the English version of the Wound QoL from the German Center for Health Services Research in Dermatology in Hamburg. We did the hard work to get it turned into a quality measure within our QCDR for PQRS reporting, and got it approved by CMS. We then programmed it as an electronic clinical quality measure (eCQM) so it could be installed into any electronic health record and posted that programming (worth about $100,000) on the internet for free so that any certified EHR could use it free of charge. We even programmed it into several electronic tablets. However, it is hard to push initiatives like this with absolutely no, and I mean ZERO funding. I personally gave it to 400 patients (only 100 both before and after treatment). Then, I provided the quality of life data along with all the patient’s wound parameters and outcome data to Professor Augustin and his staff in Hamburg so that they could try to validate the tool clinically. However, currently they don’t have funding to analyze the data. As far as I know, the data that is just sitting on someone’s computer.
Sadly, this year, CMS rejected the USWR Wound Care Quality of Life quality measure, so it didn’t make into the USWR MIPS registry. CMS officials told me that we could try to get a new quality of life measure approved in the future, but only IF we established a goal for a certain level of improvement in quality of life in response to treatment. In other words, simply administering the quality of life questionnaire to patients could not be considered a quality measure. So, that means it takes money to buy the tablets, time for the staff to give the questionnaire, organizational effort to make sure the patients get it at least 2 times, and then money to analyze patient performance, all in order to HOPEFULLY have some percentage improvement in quality of life – when the clinician can get the same quality measure “benefit” under MIPS with medication reconciliation or measuring BMI. Thus, I completely understand why no one (except me, apparently) is crazy enough to do all of that for nothing.
Nevertheless, I would like to go on record as saying that the USWR did this alone. We could not get funding from any dressing manufacturer or from any clinical wound care organization. Yet, only a few weeks ago, a major manufacturer in need of outcome data wanted to know if we had quality of life data. My answer was not very nice and I am really sorry about my snarky response.
Everyone talks about the importance of measuring quality of life in the field of wound care. I read about it in nearly every paper on wound outcomes. However, the wound care industry had the chance to make measuring wound related quality of life part of MIPS, and it didn’t happen because the support simply wasn’t there. Anyone can talk about this stuff.
If you would like to know what the immediate impact of losing the quality of life measure, consider that if you have a patient on palliative wound care, and in order to satisfy the Novitas LCD, you would like to say that “optimizing” or “improving quality of life” is the goal of your treatment, you are going to have a hard time measuring it without a validated tool for wound care. Additionally you won’t be able to get credit for measuring wound related quality of life as a quality measure under MIPS. Thus, you might not want to select that as your goal of treatment for palliative care.
So, do yourself a favor, and don’t ask me about this topic, unless you want to ask me how to make out the check you are writing to fund the next attempt by the USWR at getting CMS to approve a quality of life measure. The USWR is a 501(c)(3) non-profit organization and donations are tax deductible. I don’t receive any compensation for all the time I devote to it, nor do many of the individuals who perform the vital work necessary to ensure that this registry is available to facilitate quality reporting for wound care practitioners.

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.