The Wound Care Evidence Summit will take place in Washington, D.C. April 1-2. If you are involved in this area, you need to register now because spots are limited. The meeting will convene commercial and government payer medical directors, the FDA, NIH senior staff, wound care researchers, manufacturers, and policymakers. Here’s a link to the agenda. I thought I’d explore some of the issues that have troubled me since I got into this field.
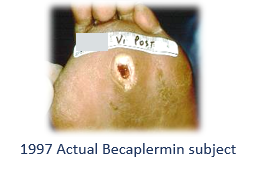
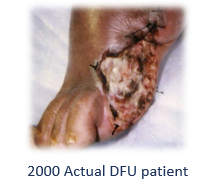
In about 1997, I was one of several investigators in the Becaplermin (REGRANEX®) Gel of randomized, controlled trial (RCT) of Diabetic foot ulcers (DFUs). That trial (which had many comorbid disease exclusion criteria) enrolled mostly Wagner 1 DFUs and Wagner Grade 2 DFUs (as long as the Wagner 2 DFUs had no exposed tendon, capsule or bone – which means, not really Wagner 2 DFUs). The 1998 trial of Apligraf had almost identical criteria. Here are some photos I dug up of actual subjects I enrolled in those trials, compared to a typical patient in my clinic:
Maybe you can see why, by 2002 I was done with RCTs. In 2017 we used a consortium of 6 clinics that participated in the US Wound Registry (USWR) to compare actual patients with DFUs to those enrolled in RCTs performed in those same clinics. The 6 clinics treated 2,634 DFUs and also enrolled 244 DFU subjects in 4 RCTs of cellular and/or tissue based products (CTPs).
Here’s how the real world patients differed from subjects enrolled in CTP RCTs:
- 12.2% of real DFU patients had renal failure – a condition excluded from all RCTs
- Among real world patients, there were 4.3 DFUs per patient, but the RCTs enrolled only 1 ulcer per patient and didn’t even record how many there were present
- 43.6% of real world patients had ulcers that were Wagner 3 or worse ulcers whereas RCTs enrolled Wagner 1 and 2 ulcers (as long as the Wagner 2 ulcers were not too bad)
- Among real patients, the initial DFU ulcer surface area was 3 times larger than ulcers in the RCTs
When we applied the Wound Healing Index, our risk stratification model, to these DFUs, the real world DFUs were statistically much less likely to heal than those enrolled in the RCTs (68.6 vs. 88.1). The WHI confirmed that the subjects enrolled in the RCTs of cellular products bore no resemblance to the typical DFU patient. This makes RCT data virtually worthless for assessing the potential value of a CTP among real world patients.
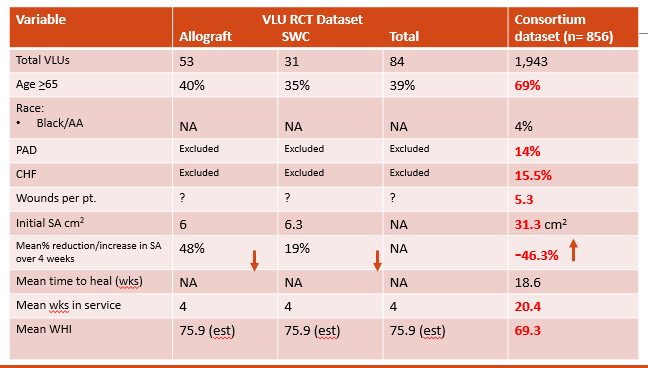
The story was the same for venous leg ulcers (VLUs). Real VLU patients have multiple ulcers that are huge, the patients are more likely to be elderly, they take 4 or 5 times longer to heal than the duration of a typical RCT, many real world VLUs get worse during treatment, and co-morbid conditions that are excluded in RCTs are COMMONLY present in real world patients.
As I have discussed in previous blogs, the result is that payers craft coverage criteria which exclude these CTPs from use in real world patients. And if that doesn’t make you angry, you haven’t been in this business long enough. See you in D.C. in April.
Serena TE, Fife CE, Eckert KA, Yaakov RA, Carter MJ. A New Approach to Clinical Research: Integrating Clinical Care, Quality Reporting, and Research Using a Wound Care Network-based Learning Healthcare System. Wound Repair Regen. 25(3): 354-365, 2017.

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.





Almost 20 years of full-time wound care and you bet I am, and have been angry for years. RCT with that much emphasis on control is BS.