The Wound Care Evidence Summit will take place May 19-20, 2022 at the Hyatt Regency Bethesda, MD. If you are involved in the field of wound care (e.g., Chief Medical Officers, researchers, Regulatory Affairs staff, Clinical Association representatives, manufacturers, etc.) you need to register now because spots are limited. The meeting will convene commercial and government payer medical directors, the FDA, NIH senior staff, wound care researchers, manufacturers, and policymakers. You can register here.
Here’s why I care about this so much: In about 1997, I was one of several investigators participating in the prospective clinical trial of Becaplermin (REGRANEX®) Gel for Diabetic foot ulcers (DFUs). That trial enrolled mostly Wagner 1 DFUs and Wagner Grade 2 DFUs (as long as the Wagner 2 DFUs had no exposed tendon, capsule or bone – which means, not really Wagner 2 DFUs). In addition to studying only relatively superficial DFUs, the trial excluded almost every serious comorbid disease except diabetes, as did the 1998 trial of Apligraf for DFUs.
Here are some photos I dug up of actual subjects I enrolled in those trials, compared to a typical patient in my clinic:

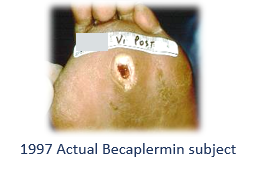
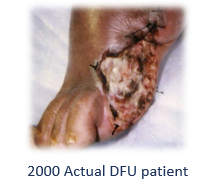
Back in 1997 and 1998 (before the Internet), I had to advertise on the Houston radio and in the newspaper to recruit subjects with a diabetic foot ulcer who were healthy enough to participate in the trials. The patients in my busy clinic had too many co-morbid conditions and their wounds were too severe, so I couldn’t recruit actual wound center patients for the studies.
The disconnect between the subjects enrolled in clinical trials and the actual patients I saw bothered me a lot. In 2009, Dr. Marissa Carter and I looked at the exclusion criteria from over 1000 clinical trials in wound care and compared them to the prevalence of co-morbid disease in several thousand real-world patients in the U.S. Wound Registry (USWR). We found that almost 75% of actual wound center patients would have been excluded from every major wound healing trial, based on their co-morbid diseases, before anyone even evaluated the severity of the wound. In fact, the subjects in 3 out of the 4 biggest prospective wound healing trials were healthier than the “man on the street.” I started to call prospective RCTs “show girl trials.”
In 2017, Dr. Tom Serena and I looked at a consortium of 6 clinics that participated in the USWR and compared the actual patients with DFUs and VLUs to the subjects enrolled in RCTs being carried out in those same clinics.
Here’s how the real world DFU patients differed from subjects enrolled in RCTs:
- 43.6% of real world patients had ulcers that were Wagner 3 or worse ulcers, whereas RCTs enrolled Wagner 1 and 2 ulcers (as long as the Wagner 2 ulcers were not too bad).
- Among real patients, the initial DFU ulcer surface area was 3 times larger than ulcers in the RCTs.
- 12.2% of real DFU patients had renal failure – a condition excluded from all RCTs.
- Among real world patients, there were 4.3 DFUs per patient, but the RCTs enrolled only 1 ulcer per patient and didn’t even record how many there were present.
The story was the same for venous leg ulcers (VLUs). Real VLU patients have multiple ulcers that are huge, the patients are more likely to be elderly, they take 4 or 5 times longer to heal than the duration of a typical RCT, many real world VLUs get worse during treatment, and co-morbid conditions that are excluded in RCTs are COMMONLY present in real world patients with VLUs.
When we applied the Wound Healing Index, a model that predicts the likelihood of healing, we found that the DFUs and VLUs enrolled in clinical trials might have been predicted to heal anyway, whereas the patients with those problems were a lot less likely to heal on their own. To make matters worse, payers craft coverage criteria for CTPs that mirror the exclusion criteria in the clinical trials. This means that CTPs aren’t “covered by insurance” for most real world DFUs and VLUs.
Lately a few brave companies have started enrolling sicker patients with more severe wounds in their clinical trials. Hats off to them! With these few exceptions, we have spent millions of dollars to demonstrate that CTPs are effective in relatively easy to heal wounds, and real-world patients cannot get those products due to coverage issues. If that doesn’t make you angry, you haven’t been in this business long enough. See you in Bethesda in May.

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.



2009: actually 17 “hi tech” RCTs, Caroline.
So true! Unfortunately, many of the providers who evaluate and use these products as well as the government that pays reimbursement for use are none the wiser. This is because actual understanding of Woundology has no formally recognized place in who can deliver wound care!! To most it’s all about the money. YET,
“Quality is never an accident. It is always the result of intelligent effort.” John Ruskin
How can there be intelligent effort without understanding!??