Last week I attended the inaugural Cellular, Acellular, and Matrix-like Product (CAMP) Summit. It was a very interesting and well-run meeting. I will digress to mention the acronym “CAMP” which is gaining traction over the more awkward (and possibly obsolete) Cellular and/or Tissue Based Products (CTPs), but CTP is currently the name recognized by the ASTM standards organization. This situation is made more complex by the fact that nearly everyone including the Centers for Medicare and Medicaid Services (CMS) tends to erroneously refer to these as “skin substitutes.” It is somehow fitting that this chaotic field, we can’t even agree on a name. I do not have a dog in the fight over naming conventions. I haven’t decided what term to use because there is no way to please everyone.
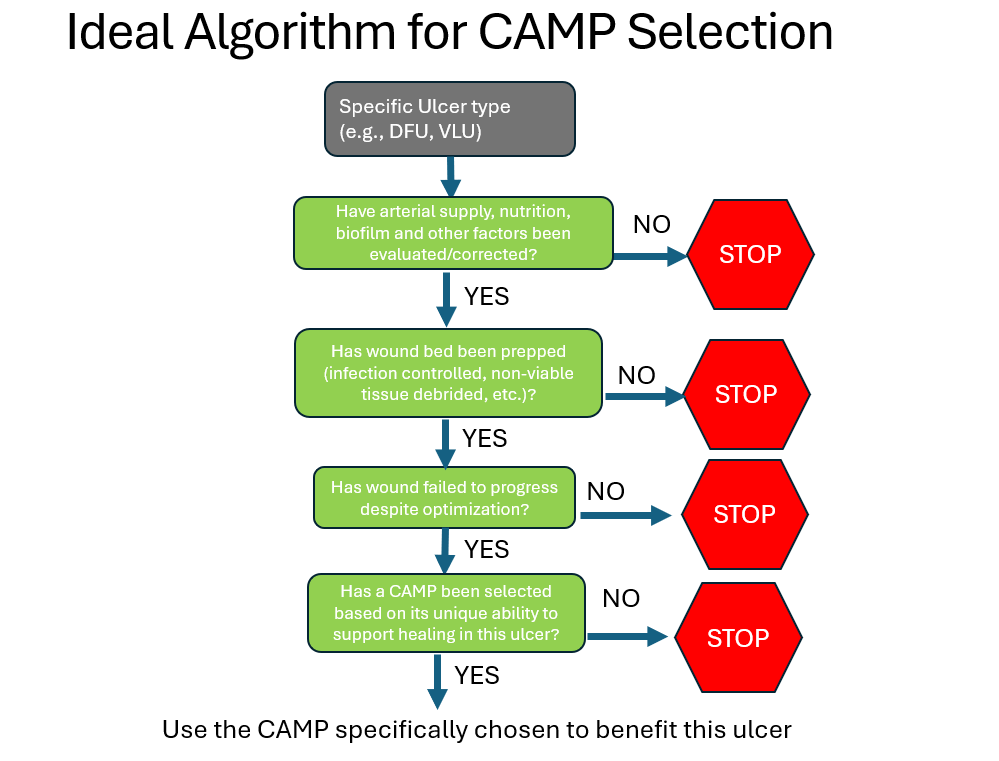
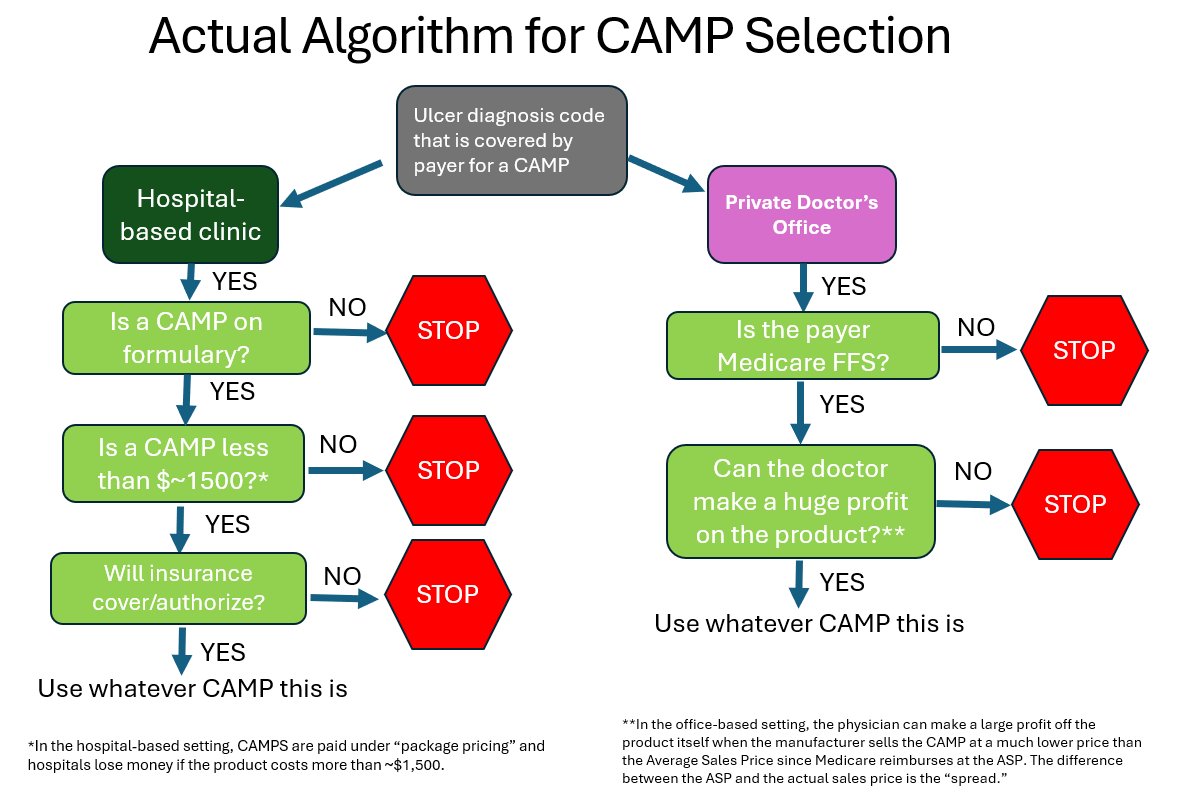
I might have more to say about the CAMP Summit, but for now I will comment only on the patient selection algorithm. In a full day of beautifully presented talks, the ideal algorithm for the use of CAMPs was discussed in detail. This includes the requirements for patient assessment, wound bed preparation, etc. I would summarize that ideal process as follows:
Additional Reading:
- More of Your Letters: Skin Substitutes, CMS, Failed Proposals and the Broken System
- How We Got Here – Answers to the Questions I Have Been Asking
- Taking a Closer Look at the OIG Report on “Skin substitute” (CTP) ASP Transparency
- “We Will All Lose in the End.” Let’s Discuss the Moral Hazard of the CTP (Skin Substitute) Industry
- A Problem Well-Stated is a Problem Half Solved… (Hopefully)

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.