Guest Blog by Michael J. Crouch, CPC, CPMA, CHT-ADMIN
Dr. Fife –
In March, I provided feedback on a skin substitute graft (SSG) audit in which I was involved that was similar to the one Martha Kelso described. The Unified Program Integrity Contractor (UPIC) required my client to provide 26 specific items as part of their review. After 15 months, and several appeal levels, that audit process finally concluded with a favorable Administrative Law Judge (ALJ) decision, so now I can provide more details.
I thought a review of the arduous journey might prove useful to your audience. I have included a timeline of the events as they transpired. I also numbered each event and have provided additional details on each step. I have also listed each auditors’ reasons for denying the claims. I hope that everyone will take the time to read the reasons for the denials issued by the UPIC, MAC and QIC because their implications could threaten the entire skin substitute industry.
Detailed Explanation of the Above Events:
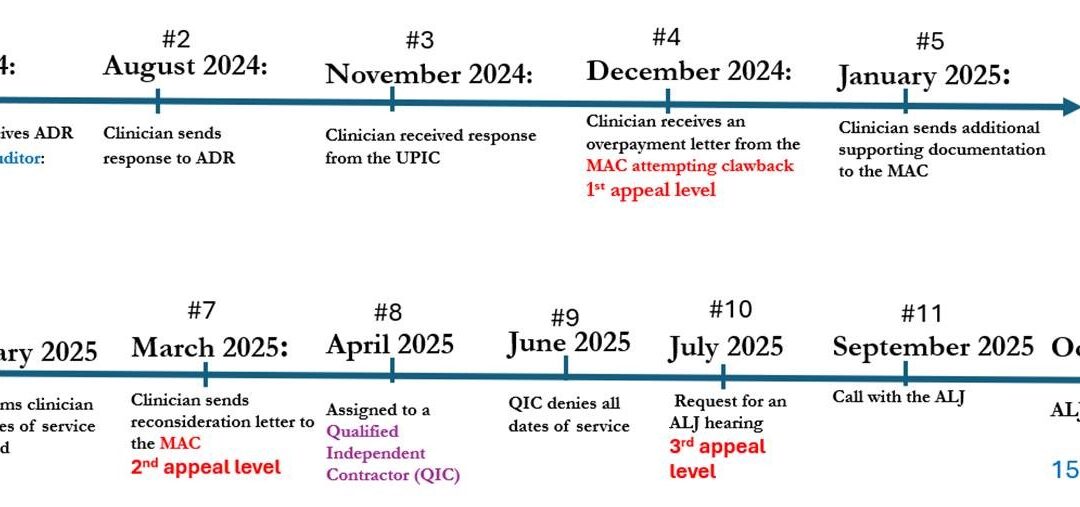
- July 2024: Clinician engaged my consulting services for assistance in responding to an Additional Documentation Request (ADR) received from a UPIC for the application of SSGs (also known as Cellular and/or Tissue-based Products).
- August 2024: Consultant conducted an audit of 12 patient records, which included the 30 dates of service requested by the UPIC, collected the documentation required, and responded to the ADR deadline.
- November 2024: Clinician received a response from the UPIC indicating that ALL dates of service were denied – – see denial reasons listed below.
- December 2024: Clinician received an overpayment letter from the MAC attempting to claw back almost $620,000. Rebuttal and Redetermination letters were sent to the MAC as part of the 1st appeal level.
- January 2025: Consultant sent additional supporting documentation to the MAC and developed cover letters for each patient account detailing specific responses for each UPIC denial reason.
- February 2025: Clinician received a response from the MAC indicating that ALL dates of service were denied – – see denial reasons listed below.
- March 2025: Consultant sent a Reconsideration letter to the MAC as part of the 2nd appeal level, along with supporting peer-reviewed and evidence-based publications (10 scientific papers) validating the use of these products as skin substitute grafts for wound healing.
- April 2025: Assigned to a Qualified Independent Contractor (QIC) who was responsible for conducting the second review.
- June 2025: Clinician received a response from the QIC indicating that ALL dates of service were denied – – see denial reasons listed below.
- July 2025: Consultant requested an ALJ hearing as part of the 3rd appeal level.
- September 2025: ALJ hearing during which counter arguments were presented for not only the QIC decision, but also the UPIC and MAC decisions. Note that none of these contractors decided to participate in the call.
- October 2025: Clinician received the ALJ decision in which it was determined that 84% of the patient accounts in question were deemed appropriate and covered, meaning the clinician kept almost $520,000 of the contested amount.
REASONS FOR DENIAL OF CHARGES AT EACH LEVEL (be sure to read these!)
A. Unified Program Integrity Contractor (UPIC) Denial Reasons:
- Records did not support that the services met, and did not exceed, the beneficiary’s medical need.
- Records failed to show comprehensive wound treatment plans to support the skin substitute therapy.
- Records failed to show consistent improvement in wounds after repeated application of skin substitutes.
- There was a pattern of records that did not show measurements, history, or detail of the initial wound to provide baseline data prior to initiation of the skin substitutes.
- There was a pattern of records that showed inconsistent units of product applied between the documentation in the procedure notes and the units billed on the claims.
- Records failed to show appropriate sizes of skin substitute product applied to the wounds. There was a pattern of records that showed the products did not cover the wounds, and a pattern of larger sizes used with no documented wastage.
B. Medicare Administrative Contractor (MAC) Denial Reasons:
- The products used are amnionic membrane allografts. The amniotic membrane acts as a covering in the fetus, provides protection, promotes gas and nutrient exchange, removes carbon dioxide, and produces hormones. Therefore, their use as a skin substitute graft for treatment of an ulcer or wound is a non-homologous use.
- These products do not have FDA approval under the Federal Food, Drug, and Cosmetic Act and or section 351 of the PHS Act. In addition, they have insufficient peer reviewed, evidence-based literature to support their use for ulcer or wound treatment or healing. They are investigational and experimental and have insufficient peer-reviewed, published evidence or any evidence-based recommendations by a specialty society or organizations to support their safety, efficacy, or clinical utility, or frequency of use as ulcer or wound healing products. (An interesting side note: Although the MAC determined that none of the products used were “approved” for use as a skin substitute, one of the products denied is on the “approved list” of skin substitutes in the pending future LCDs.)
- CPT codes 15271 and 15272, which were billed for these services, specify that products used as skin substitute grafts must provide scaffolding for cell growth and there is no literature-based evidence which demonstrates they provide skin scaffolding.
C. Qualified Independent Contractor (QIC) Denial Reasons:
- The QIC finds that these services are experimental and investigational as denoted by the Q code status nomenclature. Under Medicare law, no payment can be made under Medicare for any expenses incurred for items or services that are considered investigational and are not considered reasonable and necessary.
- Further, although the provider cited the LCD L35041 for Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds, the QIC has determined the correct policy is the MPIM, Chapter 3, Section 3.6.2.2 and Section 1862 of the Act. Since the services (Q4158, Q4229, Q4236, Q4267, and Q4276) are considered investigational and not payable as per CMS, it is the QIC’s determination that these services do not meet the medically reasonable and necessary requirements of Section 1862(a)(l)(A) of the Act.
Stay tuned for a list of “Lessons Learned” and “Other Considerations” in Part Two – – coming soon!
Michael J. Crouch, CPC, CPMA, CHT-ADMIN
michaelcrouch@cplushealthcareconsulting.com
www.cplushealthcareconsulting.com

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.



Something tells me Q4158 was not the problem and the others probably were…
Just to clarify: This provider was audited for 30 dates of service, for which he/she received $620,000? Am I reading that correctly? if so, the provider was actually paid > $20,000 per application? If true, that is absurd.
Its a lot, but you they may have been dealing with very sizable ulcers.