
by Caroline Fife, M.D. | Dec 13, 2024 | Cellular / Tissue-Based Products
CGS J15 Webinar on Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers Join the CGS Chief Medical Officer on Friday, December 20, 2024, from 10:00AM to 11:00AM Eastern Time to review the following...

by Caroline Fife, M.D. | Dec 12, 2024 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
Health and Human Services (HHS) announced Active Work Plan items (e.g., OIG audits, evaluations, and inspections) that are underway or planned over the next 2 years. Investigations regarding various aspects of “skin substitute” use are on the list along with the...

by Caroline Fife, M.D. | Dec 2, 2024 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
Recently someone passed on what a clinician had told them about the Cellular Tissue Products / Skin Substitutes that they were using. The clinician said they were not worried about being paid for a particular product because it had an established ASP (Average Sales...

by Caroline Fife, M.D. | Nov 25, 2024 | Cellular / Tissue-Based Products, CTP / Skin Substitutes, Health Information Technology and Wound Care
You should read the entire LCD, because there is a lot to unpack. I will tackle various topics over the next several weeks, but here’s the list of covered and not-covered CTPs/skin subs for diabetic foot ulcers (DFUs) and Venous Leg Ulcers (VLUs). When will the...

by Caroline Fife, M.D. | Nov 22, 2024 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
On 12/10/2024, CGS (Cigna Government Contractors) will host a multi-jurisdictional Town Hall meeting to discuss the recently released final LCD(s) for Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg...

by Caroline Fife, M.D. | Nov 14, 2024 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
Read them here (more to come): LCD Cellular Tissue Products View on CMS Website Caroline Fife, M.D.Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my...

by Caroline Fife, M.D. | Nov 6, 2024 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
CMS has published the final rule for the CY2025 Medicare Physician Fee Schedule (MPFS). As we knew from the proposed rule, there are no major changes in the payment for Cellular Tissue Products (CTPs) / skin substitutes in 2025 — although CMS stated they the...
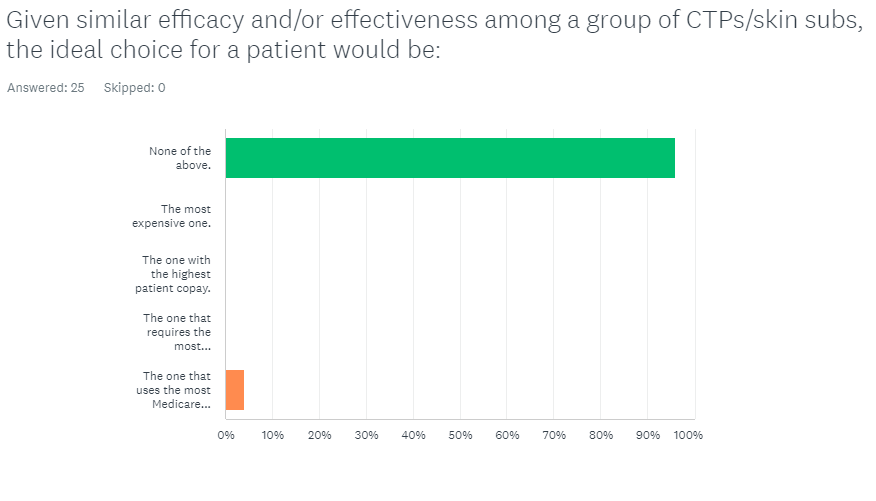
by Caroline Fife, M.D. | Oct 31, 2024 | Cellular / Tissue-Based Products, CTP / Skin Substitutes, Fight the Good Fight, Quiz / Survey
The results are in! Given similar efficacy and/or effectiveness among a group of CTPs/skin subs, the ideal choice for a patient would be: A. The most expensive one. B. The one with the highest patient copay. C. The one that requires the most applications to be...

by Caroline Fife, M.D. | Oct 25, 2024 | Cellular / Tissue-Based Products, CTP / Skin Substitutes, Fight the Good Fight, Quiz / Survey
Create your own user feedback survey Caroline Fife, M.D.Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel...

by Caroline Fife, M.D. | Oct 15, 2024 | Cellular / Tissue-Based Products, CTP / Skin Substitutes
It has taken months for me to understand that the immediate threat from CTP/skin sub over/improper use is to Accountable Care Organizations (ACOs). I’ve blogged previously about why. An ACO that asked not to be named gave me the following figures around CTP billing in...












