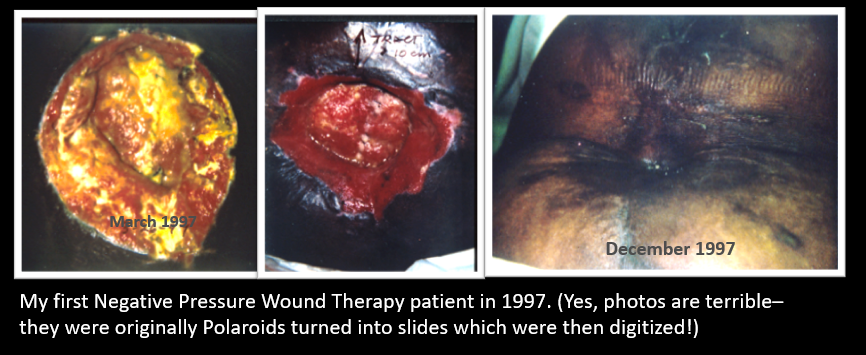
Do you still have the photos of the first patient you treated with the VAC? Here are the 1997 photos of my first VAC patient, only 2 years after the device was cleared by the FDA. I know the photo quality is terrible – they were originally Polaroids! My point is that I have kept the photos of this case for 22 years. She had an abdominal dehiscence so massive that I could bury my forearms into the area of undermining along the sidewalls of her midline defect. The first time I changed her “VAC” dressing I knew that the entire field of wound care had changed. I had been running a wound center for 7 years by then. Most of the time, I could do little more than the 16th century barber-surgeon, Ambroise Paré, who said, “I dress the wound and God heals them.”
In 1997, with negative pressure wound therapy (NPWT), it became possible to actually harness the healing process. I wish I had a photograph of the way one innovative patient created the first “portable” NPWT unit by attaching the heavy device to a skateboard with bungee cords so he could pull the unit behind him, plugged into the wall electrical outlet via a long orange industrial power cord.
The recent analysis of 2014 Medicare claims data by the Alliance of Wound Care Stakeholders demonstrated that the dehisced surgical wound is the most common chronic wound among Medicare beneficiaries. The same analysis showed that dehisced surgical wounds are also the most expensive chronic wound, with most of the costs accruing in the outpatient setting. I am willing to bet that NPWT is responsible for a lot of those costs. As the anticipated 2024 bankruptcy of the Medicare trust fund approaches, there is increasing interest in finding answers to questions like when is the optimal time to discontinue NPWT? What is the ideal suction pressure? Which patients really need incisional NPWT? Some wounds that receive NPWT likely could heal without it, but which ones?
In 2009 (yes, a decade ago) KCI was alone among wound care manufacturers in understanding the need for a risk stratification system. It partnered with the US Wound Registry and Susan Horn, PhD, of the Institute for Clinical Outcomes Research (ICOR) to fund such a project. We had hoped to create one model that worked for all wound types but, because different factors affected different wound types, 7 models were ultimately developed, one for each major ulcer category. Termed the Wound Healing Index (WHI), the nearly four-year project involved analyzing the structured data from almost 70,000 wounds in the USWR, identifying individual factors associated with failure to heal, creating predictive models and validating them from additional data.
The WHI makes it possible to predict with reasonable accuracy, at the conclusion of the first visit, whether a wound of a given type is likely to heal with standard wound treatment alone. Using the WHI, it is not necessary to wait weeks to establish a wound healing “trajectory.” The WHI allows the early identification of patients who will likely need an advanced therapy like NPWT to achieve healing, or perhaps more importantly, those who do not, allowing better targeting of healthcare resources. It is interesting to consider that despite all the hype about using artificial intelligence (AI) in wound care by analyzing photographs, there is no apparent interest in using the “actual intelligence” currently available in the form of predictive models.
The most important impact of the WHI is to enable the honest reporting of healing rates to Centers for Medicare and Medicaid Services (CMS). A recent systematic analysis found that the vast majority of hospital based wound centers publicly report healing rates better than 92% in less than four weeks. The contradiction between nearly perfect healing rates and the multibillion-dollar cost of treating chronic wounds creates a looming crisis for the field of wound care. The majority of practitioners are now subject to the Merit Based Incentive Payment System (MIPS) under which a practitioner’s Medicare Part B payments can be assessed either a bonus or a penalty based on a complex formula that includes quality performance (e.g., outcomes reporting) and per patient spending. Under MIPS, CMS requires that patient outcomes be reported using “risk stratification.” Virtually every medical specialty has developed a risk stratification system so that physicians taking care of the sickest patients would not appear to have worse outcomes than their peers.
Given the realities of the QPP, it’s time for wound care practitioners to think more like oncologists. Reputable cancer centers do not report that they cure 92% of all cancers. Thanks to the transparent and uniform way that oncologists report outcomes, we know that whether patients are likely to be cured of their cancer is determined in large part by the type and stage of their cancer. The higher mortality rate of more aggressive cancers is not interpreted as an indictment of the oncologist, but an argument for developing better treatments.
Wound care practitioners should brag about their healing rates in relation to the predicted likelihood of healing. We should be reporting the outcome of the most difficult patients, not the easiest ones, if we want to justify the need for our services and the cost of care.
In March of 2017, a few forward-thinking wound care practitioners reported the healing rate of diabetic foot ulcers and venous leg ulcers to CMS as part of their MIPS participation. These healing rate measures were stratified by the WHI. They didn’t report healing rates of over 92%. Practitioners reporting the venous leg ulcer and diabetic foot ulcer healing rate quality measures developed by the USWR reported honest healing rates to CMS. In some cases, “honest” healing rates reported to CMS under the Merit Based Incentive Payment Program (MIPS) were only 60%, because the patients were so complex. CMS still rewarded those practitioners with the highest number of points possible under MIPS for a specific measure. The reason is that the score was not determined by the percent of ulcers that healed, but whether they healed ulcers that were predicted NOT to heal. Isn’t that a better way to measure quality?
Although we all think of “the VAC” as the most important accomplishment of the company previously called KCI, history will probably show that enabling the development of the WHI was just as important to the field of wound care. Last week a private payer denied NPWT to yet another of my patients with a dehisced surgical wound. She has a genetic disease that affects collagen production, and adult onset diabetes. Using the WHI, I can prove her wound probably won’t heal without NPWT, but sadly, using the WHI to justify the need for NPWT hasn’t been promoted, even though that’s the very reason the WHI was created.
I predict that it will become harder and harder to get NPWT approved by private payers, and that includes patients with Medicare Advantage. Without using the WHI to justify the need for advanced therapeutics like NPWT, the future of wound care could look like it did in the early 1990s: dressing the wound and praying for patients to heal.
Related contents:
- Read the full article in my editorial in “Today’s Wound Clinic”
- Why Publicly Reported Healing Rates are Pure Fiction
- Another Perspective on Damn Lies and Healing rates
- Making the Right Thing the Easy Thing

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.



You are running into a barrier created partly by a lack of reporting on exactly how long it takes complex wounds to heal and what are the economic considerations with respect to same. I am aware of systematic analyses calling into question whether NPWT is cost effective etc. For the dehisced abdominal wall following surgery I believe that it is because the alternative is a) keeping the patient in hospital longer or b) spending more time undertaking dressings when the patient is out of hospital. Plus NPWT with the mesh traction technique is revolutionising management of the open abdomen. However it is not always straight forward managing complex patients as we have found https://www.hindawi.com/journals/crit/2019/2452857/