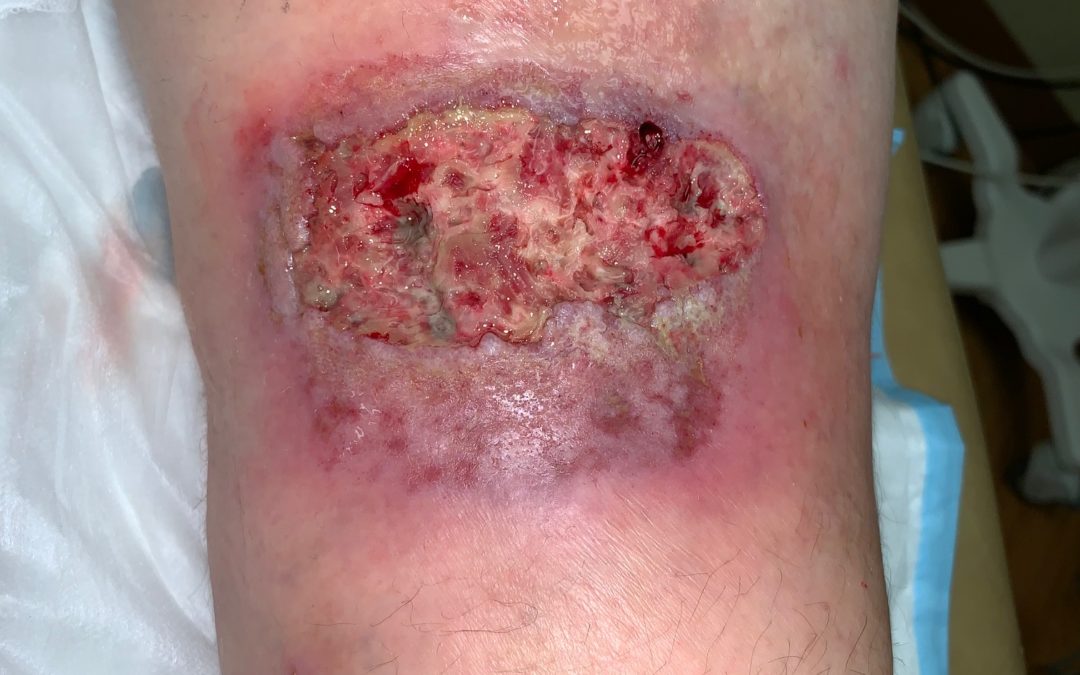
Pyoderma gangrenosum (PG) is an inflammatory skin disorder that causes excruciatingly painful skin ulcerations. It’s supposed to be rare, but it’s only rarely diagnosed. At any given time, I will have 8 or 10 patients with PG in my practice. Biopsies fail to diagnose it more than 70% of the time, but the patients all have a history of “pathergy,” dramatic worsening after minor trauma.
If you or any of your patients have been diagnosed with Pyoderma Gangrenosum (PG)- there’s hope. Dr. Alex Ortega at Oregon Health and Science University in Portland, Oregon is conducting a clinical research trial to determine the safety and efficacy of Baricitinib (Olumiant) combined with a prednisone taper for the treatment of PG.
Baricitinib suppresses a pathway of proteins that have been shown to be overabundant in PG lesions, and that play a large role in the chronic inflammation that drives PG. Baricitinib has already been approved by the FDA to treat rheumatoid arthritis, a common condition among patients with PG, which shares many similarities to the inflammatory profile of PG. Several studies are testing Baricitinib’s effectiveness in other chronic skin conditions, but this is the first and only trial testing Baricitinib in PG.
For more information, and to see if you or one of your patient’s will qualify, please contact Morgan Vague at vague@ohsu.edu.
Get this! Patients are reimbursed between $75-175 per visit. Travel reimbursement of up to $650 per visit is available for patients who live outside of Oregon or >200 miles from Oregon Health and Science University.
Here’s the patient recruitment flyer. Pass it on!
8.26.2022_PG-recruitment-flyer_patientAdditional Reading:
- The Many Faces of Pyoderma Gangrenosum – This is Not a Pressure Injury
- Pyoderma Gangrenosum, PASH, and Playing the Odds
- Hidden in Plain Sight: Pyoderma Gangrenosum
- Pyoderma Gangrenosum (PG) Hiding in Plain Sight
- Pyoderma Gangrenosum (PG): Not So Rare, and When in Doubt, Take a History
- The Many Faces of Pyoderma Grangrenosum – a Leg Ulcer Present for a Year

Dr. Fife is a world renowned wound care physician dedicated to improving patient outcomes through quality driven care. Please visit my blog at CarolineFifeMD.com and my Youtube channel at https://www.youtube.com/c/carolinefifemd/videos
The opinions, comments, and content expressed or implied in my statements are solely my own and do not necessarily reflect the position or views of Intellicure or any of the boards on which I serve.